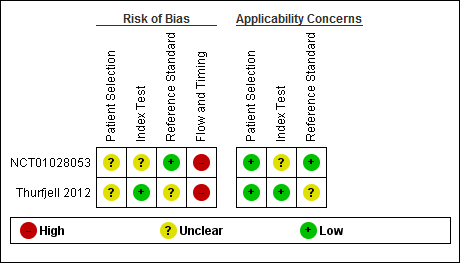
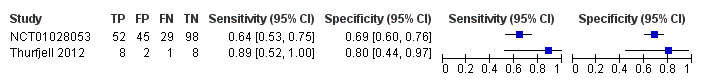Related content
Related reviews and protocols
Gabriel Martínez, Robin WM Vernooij, Paulina Fuentes Padilla, Javier Zamora, Xavier Bonfill Cosp, Leon Flicker | 22 November 2017
Shuo Zhang, Nadja Smailagic, Chris Hyde, Anna H Noel‐Storr, Yemisi Takwoingi, Rupert McShane, Juan Feng | 23 July 2014
Nadja Smailagica, Marco Vacante, Chris Hyde, Steven Martin, Obioha Ukoumunne, Christos Sachpekidis | 28 January 2015
Craig Ritchie, Nadja Smailagic, Anna H Noel‐Storr, Yemisi Takwoingi, Leon Flicker, Sam E Mason, Rupert McShane | 10 June 2014
Craig Ritchie, Nadja Smailagica, Anna H Noel‐Storr, Obioha Ukoumunne, Emma C Ladds, Steven Martin | 22 March 2017
Gemma Lombardi, Giada Crescioli, Enrica Cavedo, Ersilia Lucenteforte, Giovanni Casazza, Alessandro‐Giacco Bellatorre, Chiara Lista, Giorgio Costantino, Giovanni Frisoni, Gianni Virgili, Graziella Filippini | 2 March 2020
Ingrid Arevalo-Rodriguez, Nadja Smailagic, Marta Roqué-Figuls, Agustín Ciapponi, Erick Sanchez-Perez, Antri Giannakou, Olga L Pedraza, Xavier Bonfill Cosp, Sarah Cullum | 27 July 2021
Gabriel Martínez, Robin WM Vernooij, Paulina Fuentes Padilla, Javier Zamora, Leon Flicker, Xavier Bonfill Cosp | 22 November 2017
Michelle Kokkinou, Lucy C Beishon, Nadja Smailagic, Anna H Noel-Storr, Chris Hyde, Obioha Ukoumunne, Rosemary E Worrall, Anja Hayen, Meera Desai, Abhishekh Hulegar Ashok, Eleanor J Paul, Aikaterini Georgopoulou, Tiziana Casoli, Terry J Quinn, Craig W Ritchie | 10 February 2021
Jennifer K Burton, David J Stott, Rupert McShane, Anna H Noel-Storr, Rhiannon S Swann-Price, Terry J Quinn | 18 July 2021