术后激素替代疗法用于上皮性卵巢癌
Information
- DOI:
- https://doi.org/10.1002/14651858.CD012559.pub2Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 28 January 2020see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Gynaecological, Neuro-oncology and Orphan Cancer Group
- Copyright:
-
- Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
Protocol development: NS, EM, TL
Selection of studies: NS, RB
Assessing eligibility of studies: NS, RB; third person: TL
Data extraction: NS, KP; third person: RB
Data entry into Review Manager 5: NS, TL; spot‐check: KP
Assessment of risk of bias: NS, RB; third person: EM
Analysing data: NS, TL
Drafting the final review: NS, RB, KP, TL, EM
Updating the review: NS, RB, KP, TL, EM
Sources of support
Internal sources
-
Faculty of Medicine, Prince of Songkla University, Thailand.
External sources
-
Thailand Research Fund (Distinguished Research Professor Award), Thailand.
Declarations of interest
Nungrutai Saeaib: none known
Krantarat Peeyananjarassri: none known
Tippawan Liabsuetrakul: none known
Rakchai Buhachat: none known
Eva Myriokefalitaki: none known
Acknowledgements
We would like to thank Jo Morrison for clinical and editorial advice, Jo Platt for designing and running the searches and Gail Quinn, Clare Jess and Tracey Harrison for their valuable contribution to the editorial process.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers Group. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, National Insititute for Health Research (NIHR), National Health Service, or the Department of Health.
We thank the referees for many helpful suggestions and comments; these referees included Fani Kokka, Elly Brockbank, Ruth Payne and Nicolette Biglia.
Version history
| Published | Title | Stage | Authors | Version |
| 2020 Jan 28 | Hormone replacement therapy after surgery for epithelial ovarian cancer | Review | Nungrutai Saeaib, Krantarat Peeyananjarassri, Tippawan Liabsuetrakul, Rakchai Buhachat, Eva Myriokefalitaki | |
| 2017 Feb 24 | Hormone replacement therapy after surgery for epithelial ovarian cancer | Protocol | Nungrutai Saeaib, Krantarat Peeyananjarassri, Tippawan Liabsuetrakul, Rakchai Buhachat, Eva Myriokefalitaki | |
Differences between protocol and review
We excluded, from further subgroup analysis, women who underwent fertility‐preservation surgery (retention of one ovary). We could not compare regimens and durations of HRT administration because of the small number of participants. Adverse events of pulmonary embolism, deep vein thrombosis and gallstones were not reported in the included studies, however we aim to analyse these results including oestrogen and progesterone receptor status in future updates of this review if appropriate data become available. We added transient ischaemic attack and cerebrovascular accident to the adverse events, and changed the method for estimating the hazard ratio from standard error using methods of Parmar 1998 to using the Observed minus Expected events (O‐E) and the Variance (V) using formula according to Tierney 2007.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Female; Humans;
PICOs
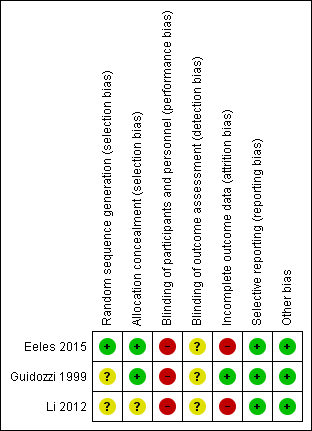
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
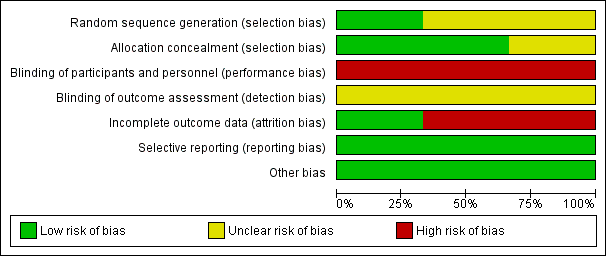
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Hormone replacement therapy versus no hormone replacement therapy, Outcome 1 Overall survival.

Comparison 1 Hormone replacement therapy versus no hormone replacement therapy, Outcome 2 Quality of life, general condition.

Comparison 1 Hormone replacement therapy versus no hormone replacement therapy, Outcome 3 Progression‐free survival.

Comparison 1 Hormone replacement therapy versus no hormone replacement therapy, Outcome 4 Incidence of breast cancer.

Comparison 1 Hormone replacement therapy versus no hormone replacement therapy, Outcome 5 Transient ischaemic attack.

Comparison 1 Hormone replacement therapy versus no hormone replacement therapy, Outcome 6 Cerebrovascular accident.

Comparison 1 Hormone replacement therapy versus no hormone replacement therapy, Outcome 7 Myocardial infarction.
| Hormone replacement therapy (HRT) compared to no HRT for epithelial ovarian cancer (EOC) | ||||||
| Patients: women of any age diagnosed with any stage of EOC who had surgical treatment, regardless of chemotherapy treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with no HRT | Risk with HRT | |||||
| Overall survival | Study population | HR 0.71 | 350 | ⊕⊕⊝⊝ | The median follow‐up time in the three included studies was 31.4 months, 90 months and 19.1 years. | |
| 795 per 1,000 | 675 per 1,000 | |||||
| Quality of life, general condition | The mean quality of life (general condition) in the no HRT group was 13.84 points. | The mean quality of life (general condition) in the HRT group was 13.67 points higher | ‐ | 75 | ⊕⊝⊝⊝ | The EORTC‐C30 questionnaire was used to evaluate this outcome; higher values correspond with improvement. |
| Progression‐free survival | Study population | HR 0.76 | 275 | ⊕⊕⊝⊝ | The median follow‐up time in the two studies was 90 months and 19.1 years. | |
| 773 per 1,000 | 676 per 1,000 | |||||
| Incidence of breast cancer | Study population | RR 2.00 | 225 | ⊕⊝⊝⊝ | The median follow‐up time in the two studies was 31.41 months and 19.1 years. | |
| 8 per 1,000 | 17 per 1,000 | |||||
| Incidence of transient ischaemic attack | Study population | RR 5.00 | 150 | ⊕⊝⊝⊝ | The median follow‐up time in the study was 19.1 years. | |
| 7 per 1,000 | 33 per 1,000 | |||||
| Incidence of cerebrovascular accident | Study population | RR 0.67 | 150 | ⊕⊝⊝⊝ | The median follow‐up time in the study was 19.1 years. | |
| 40 per 1,000 | 27 per 1,000 | |||||
| Incidence of myocardial infarction | Study population | RR 0.20 | 150 | ⊕⊝⊝⊝ | The median follow‐up time in the study was 19.1 years. | |
| 27 per 1,000 | 5 per 1,000 | |||||
| Incidence of gallstones | ‐ | ‐ | ‐ | ‐ | ‐ | The incidence of gallstones was not reported in the included studies. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level due to limitations in study design | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 3 | 350 | Hazard Ratio (95% CI) | 0.71 [0.54, 0.93] |
| 2 Quality of life, general condition Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Progression‐free survival Show forest plot | 2 | 275 | Hazard Ratio (95% CI) | 0.76 [0.57, 1.01] |
| 4 Incidence of breast cancer Show forest plot | 2 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.59] |
| 5 Transient ischaemic attack Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Cerebrovascular accident Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Myocardial infarction Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |