Fluoride supplementation (with tablets, drops, lozenges or chewing gum) in pregnant women for preventing dental caries in the primary teeth of their children
Information
- DOI:
- https://doi.org/10.1002/14651858.CD011850.pub2Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 23 October 2017see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Oral Health Group
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
Erika Ota (EO) conceived and drafted the full review. Rena Takahashi (RT) and Keika Hoshi (KH) were responsible for the selection of studies. RT and EO were responsible for data extraction and management. KH, Yoshihiro Toyoshima (YT) and EO were responsible for the risk of bias assessment. KH, Hidemichi Yuasa (HY), YT and Rintaro Mori (RM) commented on and supervised the protocol and review. All review authors read and approved the final version.
Sources of support
Internal sources
-
National Center for Child Health and Development, Japan.
Grant 26A‐5
External sources
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, the NIHR, the NHS or the Department of Health
-
Cochrane Oral Health Global Alliance, Other.
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (oralhealth.cochrane.org/partnerships‐alliances). Contributors over the past year have been the American Association of Public Health Dentistry, USA; the British Association for the Study of Community Dentistry, UK; the British Society of Paediatric Dentistry, UK; the Canadian Dental Hygienists Association, Canada; the Centre for Dental Education and Research at All India Institute of Medical Sciences, India; the National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; NHS Education for Scotland, UK; and the Swiss Society for Endodontology, Switzerland
-
Ministry of Health Labour and Welfare, Japan.
Health Labour Sciences Research Grant (No. 13800128)
-
School of Dentistry, The University of Manchester, UK.
-
The Clinical Research Program for Child Health and Development from the Japan Agency for Medical Research and Development (AMED), Japan.
Declarations of interest
Rena Takahashi: none known.
Erika Ota: none known.
Keika Hoshi: none known.
Toru Naito: none known.
Yoshihiro Toyoshima: none known.
Hidemichi Yuasa: none known.
Rintaro Mori: none known.
Eishu Nango: none known.
Acknowledgements
We would like to thank Cochrane Oral Health for their help in developing this review. We thank Valeria Marinho, Helen Worthington, Annetta Tsang and Derek Richards for their comments on the draft. We would also like to thank Emma Barber for her editorial assistance in preparing the protocol for this review, and Anne Littlewood for developing the search strategy.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Oct 23 | Fluoride supplementation (with tablets, drops, lozenges or chewing gum) in pregnant women for preventing dental caries in the primary teeth of their children | Review | Rena Takahashi, Erika Ota, Keika Hoshi, Toru Naito, Yoshihiro Toyoshima, Hidemichi Yuasa, Rintaro Mori, Eishu Nango | |
| 2015 Aug 28 | Fluoride supplementation in pregnant women for preventing dental caries in the primary teeth of their children | Protocol | Rena Takahashi, Erika Ota, Keika Hoshi, Toru Naito, Yoshihiro Toyoshima, Hidemichi Yuasa, Rintaro Mori | |
Differences between protocol and review
Fluorosis added as a primary outcome of the review. Adverse effects other than fluorosis added as secondary outcomes in the review.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Child, Preschool; Female; Humans; Infant; Pregnancy;
PICOs

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
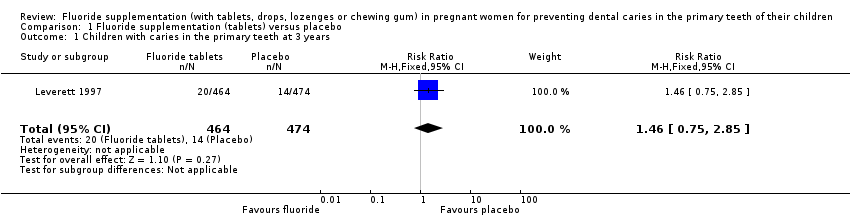
Comparison 1 Fluoride supplementation (tablets) versus placebo, Outcome 1 Children with caries in the primary teeth at 3 years.

Comparison 1 Fluoride supplementation (tablets) versus placebo, Outcome 2 Children with caries in the primary teeth at 5 years.
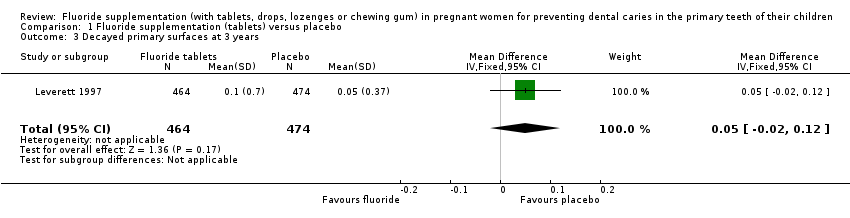
Comparison 1 Fluoride supplementation (tablets) versus placebo, Outcome 3 Decayed primary surfaces at 3 years.

Comparison 1 Fluoride supplementation (tablets) versus placebo, Outcome 4 Filled primary surfaces at 3 years.

Comparison 1 Fluoride supplementation (tablets) versus placebo, Outcome 5 Decayed or filled primary surfaces at 3 years.

Comparison 1 Fluoride supplementation (tablets) versus placebo, Outcome 6 Decayed primary surfaces at 5 years.
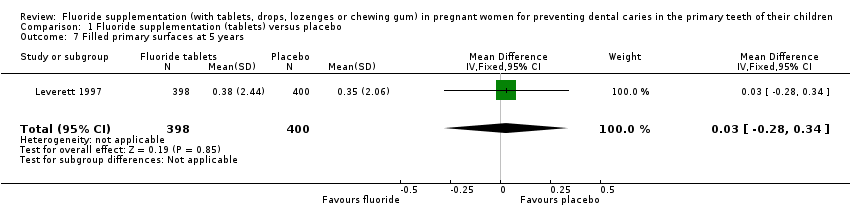
Comparison 1 Fluoride supplementation (tablets) versus placebo, Outcome 7 Filled primary surfaces at 5 years.

Comparison 1 Fluoride supplementation (tablets) versus placebo, Outcome 8 Decayed or filled primary surfaces at 5 years.
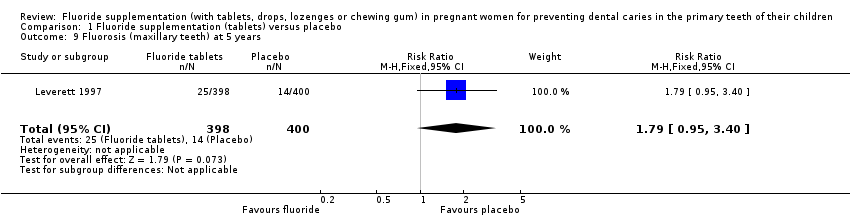
Comparison 1 Fluoride supplementation (tablets) versus placebo, Outcome 9 Fluorosis (maxillary teeth) at 5 years.

Comparison 1 Fluoride supplementation (tablets) versus placebo, Outcome 10 Fluorosis (mandibular teeth) at 5 years.
| Fluoride supplementation (tablets) compared to placebo for pregnant women for preventing dental caries in the primary teeth of their children | ||||||
| Population: pregnant women for preventing dental caries in the primary teeth of their children | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with fluoride supplementation (tablets) | |||||
| Children with caries in the primary teeth at 3 years | Study population | RR 1.46 | 938 | ⊕⊝⊝⊝ | At 5 years RR 0.84 | |
| 30 per 1000 | 43 per 1000 | |||||
| Decayed or filled primary tooth surfaces at 3 years | The mean decayed or filled surfaces at 3 years was 0.30 | MD 0.12 higher | ‐ | 938 | ⊕⊝⊝⊝ | Not significant at 5 years |
| Fluorosis (maxillary teeth) at 5 years | Study population | RR 1.79 | 798 | ⊕⊝⊝⊝ | Fluorosis in mandibular teeth at 5 years RR 0.89 | |
| 35 per 1000 | 63 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by 2 for imprecision: few events, and CI included appreciable benefits and harms. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Children with caries in the primary teeth at 3 years Show forest plot | 1 | 938 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.75, 2.85] |
| 2 Children with caries in the primary teeth at 5 years Show forest plot | 1 | 798 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.53, 1.33] |
| 3 Decayed primary surfaces at 3 years Show forest plot | 1 | 938 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.02, 0.12] |
| 4 Filled primary surfaces at 3 years Show forest plot | 1 | 938 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.07, 0.21] |
| 5 Decayed or filled primary surfaces at 3 years Show forest plot | 1 | 938 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.05, 0.29] |
| 6 Decayed primary surfaces at 5 years Show forest plot | 1 | 798 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.17, 0.05] |
| 7 Filled primary surfaces at 5 years Show forest plot | 1 | 798 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.28, 0.34] |
| 8 Decayed or filled primary surfaces at 5 years Show forest plot | 1 | 798 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.42, 0.32] |
| 9 Fluorosis (maxillary teeth) at 5 years Show forest plot | 1 | 798 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [0.95, 3.40] |
| 10 Fluorosis (mandibular teeth) at 5 years Show forest plot | 1 | 798 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.35, 2.29] |


