| Hyaluronic acid for osteoarthritis of the ankle |
| Patient or population: patients with osteoarthritis of the ankle
Settings: Rehabilitation centre / hospital
Intervention: hyaluronic acid
Comparison: placebo |
| | | | | | |
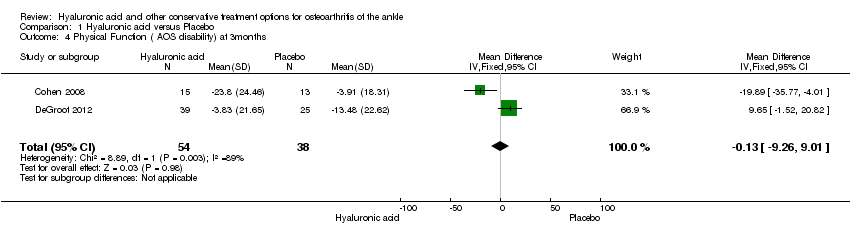
| AOS total (Pain & Physical function)
AOS total score. Scale from: 0 to 100 (0 = being no pain/disability, 100 = worst imaginable pain/disability).
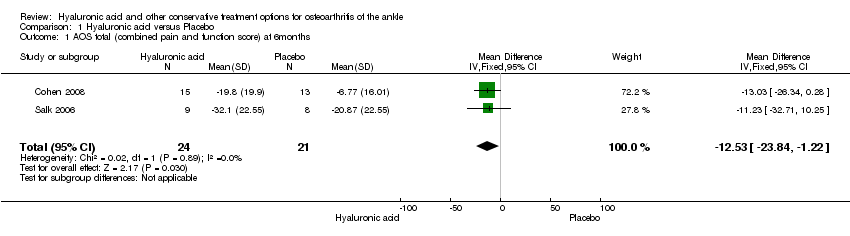
Follow‐up: 6 months | The mean pain/physical function change ranged across the control groups from 6.8 to 20.9 points lower with a weighted mean of
12.14 lower | The mean pain/physical function in the hyaluronic acid group was 12.53 points lower (23.84 lower to 1.22 lower) compared to placebo at 6 months. | | 45
(2 studies) | ⊕⊕⊝⊝
Low1 | A lower score indicates less pain and a better physical function. It is not known if a change of 12 points is clinically relevant. NNT = 4 (95% CI 2 to 205) (using a SMD = 0.5 as a minimum important difference). Absolute risk difference is −12.53% (95% CI −23.84 to −1.22). Relative percentage change is 1.85% (95% CI 0.18 to 3.58%). |
| Radiographic Joint Structure Changes | See comment | See comment | Not estimable | 0
(0) | See comment | Radiographic joint structure changes were not investigated. |
| Quality of Life SF12. Scale from: 0 to 100. Follow‐up: mean 6 months. | See comment | See Comment | Not estimable | 45 (2 studies) | See comment | Cohen 2008 only described that there was no significant difference between placebo and intervention for the SF12 outcome, no exact data was provided. Salk 2006 could not provide us with the standard deviations, so no estimate of the SF12 could be made. He demonstrated a statistically significant difference in his paper favouring hyaluronic acid at 6 months. |
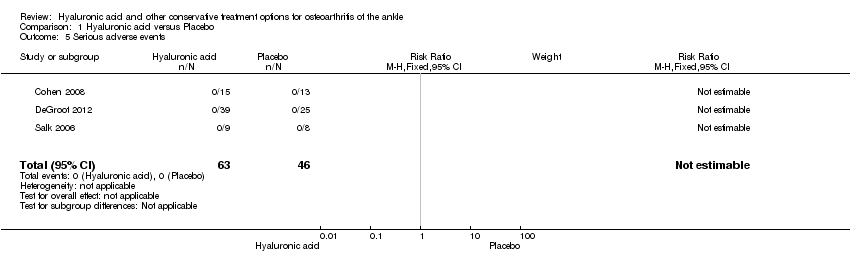
| Number of participants experiencing any serious adverse events
Follow‐up: 3 to 6 months | See comment | See comment | Not estimable | 109
(3 studies) | See comment | No serious adverse events (SAEs) were noted |
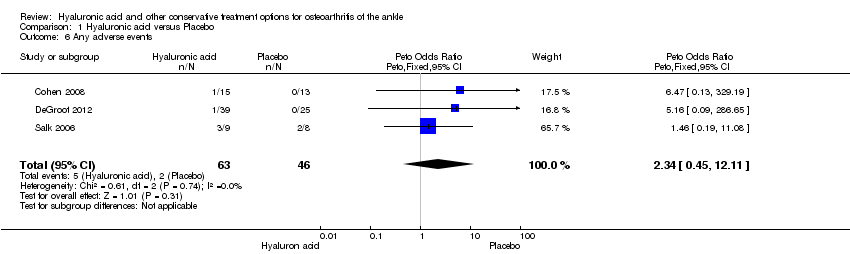
| Number of participants experiencing any adverse event
Follow‐up: 3 to 6 months | 43 per 1000 | 35 per 1000
higher (26 fewer to 241 more) compared to placebo. | RR 1.66
(0.47 to 5.88) | 109
(3 studies) | ⊕⊕⊝⊝
low1 | Peto Odds Ratio is 2.34 (95% CI 0.45 to 12.11) Absolute risk difference is 5.00% (−5 to 14), relative percentage change is 66% (−53% to 488%). Adverse events for all 3 studies were reported, even though DeGroot had a follow up of 3 months. All adverse events resolved within a week after injection, so a shorter follow up has no effect on the estimate of effect. |
| Participants who withdraw because of an adverse event or any other reason
Follow‐up: 3 to 6 months | See comment | See comment | Not estimable | 109
(3 studies) | See comment | No participants withdrew because of an adverse event |
| AOS: Ankle Osteoarthritis ScaleCI: Confidence interval; RR: Risk ratio; OR: Odds ratio; SF12: short form 12 |
| GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate. Grade criteria: study limitation, indirectness, inconsistency, imprecision, publication bias. |