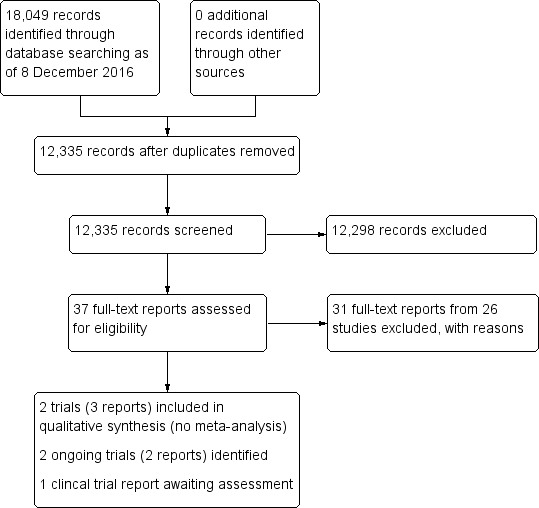
Topical medication instillation techniques for glaucoma
Information
- DOI:
- https://doi.org/10.1002/14651858.CD010520.pub2Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 20 February 2017see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Eyes and Vision Group
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
-
LX developed, designed, and wrote the protocol. XW and MW provided feedback on the protocol.
-
Conceiving and designing the review: LX.
-
Designing and undertaking search strategies: Lori Rosman (Cochrane Eyes and Vision (CEV)).
-
Screening search results: LX, XW, MW, CEV.
-
Organizing retrieval of papers: LX.
-
Screening retrieved papers against inclusion criteria: LX, XW, MW.
-
Appraising risk of bias: LX, XW, MW, CEV.
-
Extracting data from papers: LX, XW, MW.
-
Writing to authors of papers for additional information: LX.
-
Entering data into Review Manager 5: LX, XW, MW.
-
Analysis of data: LX, XW, MW.
-
Interpretation of data:
-
providing a methodological perspective: LX, XW, MW, CEV;
-
providing a clinical perspective: LX, XW, MW;
-
providing a consumer perspective: LX, XW, MW.
-
-
Writing the review: LX, XW, MW.
Sources of support
Internal sources
-
Johns Hopkins University, Baltimore, Maryland, USA.
External sources
-
Methodological support provided by the Cochrane Eyes and Vision US Satellite, which is funded by the National Eye Institute, National Institutes of Health, Grant 1 U01 EY020522, USA.
-
National Institute for Health Research (NIHR), UK.
-
Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
-
The NIHR also funds the CEV Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
-
Declarations of interest
LX: none known.
XW: none known.
MW: none known.
Acknowledgements
We acknowledge Cochrane Eyes and Vision (CEV) for assisting with the preparation of this review. We thank Lori Rosman, Information Specialist for CEV@US, for developing and executing the electronic search strategy. We thank Scott Davis, Barbara Hawkins, and the CEV editors for comments.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Feb 20 | Topical medication instillation techniques for glaucoma | Review | Li Xu, Xuemei Wang, Meijing Wu | |
| 2013 May 31 | Topical medication instillation techniques for glaucoma | Protocol | Li Xu, Xuemei Wang, Meijing Wu | |
Differences between protocol and review
We added the assessment of the certainty of evidence using the GRADE approach and a 'Summary of findings' table to the review methods. The 'Summary of findings' table was not part of the original Cochrane protocol, thus the selection of outcomes presented in the table was made post hoc. We based our selection on core outcomes for glaucoma research that have been proposed in the literature (Ismail 2016).
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Administration, Ophthalmic;
- Antihypertensive Agents [*administration & dosage, adverse effects];
- Bimatoprost [administration & dosage];
- Eyelashes [anatomy & histology, drug effects];
- Glaucoma [*drug therapy];
- Intraocular Pressure [*drug effects];
- Latanoprost;
- Ophthalmic Solutions [administration & dosage, adverse effects];
- Prostaglandins F, Synthetic [administration & dosage];
- Randomized Controlled Trials as Topic;
- Travoprost [administration & dosage];
Medical Subject Headings Check Words
Humans;
PICOs

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
| Topical medication instillation techniques for glaucoma | ||||||
| Population: participants with glaucoma or ocular hypertension Settings: ophthalmology clinics Intervention: any intervention aimed to increase effectiveness or reduce adverse events when using topical medications (e.g. eyelid closure, nasolacrimal occlusion, removal of excess fluid after instillation) Comparison: no intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No intervention | Instillation intervention | |||||
| Proportion of participants with IOP < 21 mmHg at 1 year' follow‐up | Not reported | ‐ | ||||
| Mean IOP change from baseline at 1 year' follow‐up | Not reported | 1 trial reported that reduction of IOP was similar in eyes when the eyelid was kept closed for up to 3 minutes after instillation of drops than fellow eyes that did not practice eyelid closure at 2 weeks (MD ‐0.33 mmHg, 95% CI ‐0.8 to 1.5; 51 participants); moderate‐certainty evidence.1 | ||||
| Participant‐reported outcomes related to the ease, convenience, and comfort of instillation at 1 year' follow‐up | Not reported | ‐ | ||||
| Physiologic measurements of systemic absorption at 1 year' follow‐up | Not reported | ‐ | ||||
| Escalation of therapy at 1 year' follow‐up | Not reported | ‐ | ||||
| Mean change in visual fields at 1 year' follow‐up | Not reported | ‐ | ||||
| Adverse events at 1 year' follow‐up | Not reported | 1 trial with up to 4 months' follow‐up reported that eyelashes were shorter among eyes when participants had wiped to remove excess fluid compared with fellow eyes that were not wiped (MD ‐1.70 mm, 95% CI ‐3.46 to 0.06; 10 participants) and fewer eyes had eyelash growth of > 1.5 mm when wiping compared with not wiping (RR 0.11, 95% CI 0.01 to 1.24); low‐certainty evidence.1,2 This same trial also reported that fewer eyes showed skin hyperpigmentation in the eyelid region towards the nose when wiping compared with not wiping (RR 0.07, 95% CI 0.01 to 0.84); however, the difference was not certain when assessing skin hyperpigmentation in the eyelid region towards the temples (RR 0.44, 95% CI 0.07 to 2.66) or hair growth on the skin around the eye (RR 1.00, 95% CI 0.17 to 5.98); low‐certainty evidence.1,2 | ||||
| *The basis for the assumed risk is the risk in the control group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the control group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IOP: intraocular pressure; MD: mean difference; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level for risk of performance, detection bias, or both. | ||||||
| Drug class | Ocular adverse events | Systemic adverse events |
| Alpha‐agonists (e.g. apraclonidine, brimonidine) | Allergic reactions Blurred vision Burning/stinging/discomfort Follicular conjunctival response Hyperemia Itching Photophobia | Allergic reactions Drowsiness Dry mouth Fatigue Headache Hypotension |
| Beta‐blockers (e.g. betaxolol, carteolol, levobunolol, timolol) | Allergy Blurred vision Burning/stinging/discomfort Corneal erosion Dry eyes Hyperemia Hypotony Ptosis Superficial punctate keratitis Visual disturbances | Bradycardia Depression Dizziness or light‐headedness Fatigue Headache Indigestion or heart pain Insomnia Joint pain Nausea Shortness of breath |
| Carbonic anhydrase inhibitors (e.g. acetazolamide, brinzolamide, dorzolamide) | Allergy Blurred vision Burning/stinging/discomfort Dry eyes Foreign body sensation Hyperemia Photophobia Superficial punctate keratitis | Allergic reactions Bitter or metallic taste Dizziness Fatigue Gastrointestinal distress Headache |
| Parasympathomimetics (e.g. carbachol, pilocarpine) | Blurred vision Burning/stinging/discomfort Eyelid twitching Hyperemia Itching Increased tearing Poor vision in dim light Visual disturbances | Dizziness Headache Hypoglycemia Increased saliva Increased sweating Nausea |
| Prostaglandin analogues (e.g. bimatoprost, latanoprost, travoprost) | Blurred vision Burning/stinging/discomfort Dry eyes Eyelash growth Foreign body sensation Hyperemia Increased tearing Iris/skin discoloration Itching Photophobia | Cold symptoms Exacerbation of asthma Facial rash Joint or muscle pain Upper respiratory infection |