Tratamiento periodóntico adyuvante para la infección gástrica por Helicobacter pylori
Appendices
Appendix 1. CENTRAL search strategy
-
h‐pylori.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
h‐pylori infection.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
Helicobacter pylori.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
Helicobacter pylori infection.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
PPI.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
proton pump inhibitor.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
or/1‐6
-
(clarithromycin or 6‐o‐methylerythromycin or abbott‐56268 or biaxin or clarith or klaricid or "te 031").mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
(amoxicillin or actimoxi or almodan or amix or amopen or amoram or amoxicot or amoxidin or amoxil or amoxymed or amrit or biomox or brl 2333 or clamoxyl or dispermox or flemoxin solutab or galenamox or larotid or moxatag or moxilin or p‐hydroxyampicillin or penamox or polymox or respillin or rimoxallin or senox or sumox or trimox or utimox or wymox or zoxycil).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
(2 methyl 5 nitroimidazole 1 ethanol or anabact or bayer 5360 or clont or danizol or edg dentalgel or elyzol or flagyl or gineflavir or metric or metro iv or metrocream or metrodzhil or metrogel or metrolotion or metrolyl or metronizole or metrotop or metrovex or metrozol or metryl or nidazol or noritate or norzol or nydamax or obagi or protostat or rozex or satric or trichopol or tricom or trivazol or vagilen or vaginyl or vandazole or vitazol or zadstat or zidoval).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
(chlorhexidine or acclean or acriflex or avagard or betasept or calgon vesta or chloraprep or chlorhexamed or chlorhexigard or chlorohex 2000 or chlorostat or corsodyl or curasept ads 220 or cx antiseptic dusting or denticare or dyna‐hex or eludril or excel or gibitan or habistat or hexidine or hibiclens or hibident or hibiscrub or hibisol or hibitane or mk 412a or novalsan or oris or oro clense or peridex or periochip or periogard or periosep or perisol or phiso‐med or pre‐scrub ii or rotersept or sebidin a or spectrum‐4 or sterexidine or steripod pink or tubulicid or unisept or uriflex c).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
(omeprazole or h 16868 losec or nexium or omesec or prilosec or rapinex or ulcergard or zegerid).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
(ranitidine or ah 19065 or azanplus or biotidin or gr 122311x or pylorid or raciran or raniberl or ranisen or rantec or sostril or taladine or tritec or wal‐zan or zaedoc or zantac).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
(tinidazole or bioshik or fasigin or fasigyne or tindamax or tricolam).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
or/8‐14
-
(periodontal and (disease$ or therap$)).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
(oral hygiene or buccal cavity or dental hygiene).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
periodontal scaling.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
periodontal root planing.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
subgingival bacterial plaque.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
(gingival and (pocket$ or plaque$)).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
or/16‐21
-
7 and 15 and 22
Appendix 2. MEDLINE search strategy
-
randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
randomized.ab.
-
placebo.ab.
-
drug therapy.fs.
-
randomly.ab.
-
trial.ab.
-
groups.ab.
-
or/1‐8
-
exp animals/ not humans.sh.
-
9 not 10
-
helicobacter pylori.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
helicobacter.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
pylori.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
H‐pylori.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
or/12‐15
-
PPI.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
proton pump inhibitor.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
(amoxicillin or actimoxi or almodan or amix or amopen or amoram or amoxicot or amoxidin or amoxil or amoxymed or amrit or biomox or brl 2333 or clamoxyl or dispermox or flemoxin solutab or galenamox or larotid or moxatag or moxilin or p‐hydroxyampicillin or penamox or polymox or respillin or rimoxallin or senox or sumox or trimox or utimox or wymox or zoxycil).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
(clarithromycin or 6‐o‐methylerythromycin or abbott‐56268 or biaxin or clarith or klaricid or "te 031").mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
(2 methyl 5 nitroimidazole 1 ethanol or anabact or bayer 5360 or clont or danizol or edg dentalgel or elyzol or flagyl or gineflavir or metric or metro iv or metrocream or metrodzhil or metrogel or metrolotion or metrolyl or metronizole or metrotop or metrovex or metrozol or metryl or nidazol or noritate or norzol or nydamax or obagi or protostat or rozex or satric or trichopol or tricom or trivazol or vagilen or vaginyl or vandazole or vitazol or zadstat or zidoval).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
(chlorhexidine or acclean or acriflex or avagard or betasept or calgon vesta or chloraprep or chlorhexamed or chlorhexigard or chlorohex 2000 or chlorostat or corsodyl or curasept ads 220 or cx antiseptic dusting or denticare or dyna‐hex or eludril or excel or gibitan or habistat or hexidine or hibiclens or hibident or hibiscrub or hibisol or hibitane or mk 412a or novalsan or oris or oro clense or peridex or periochip or periogard or periosep or perisol or phiso‐med or pre‐scrub ii or rotersept or sebidin a or spectrum‐4 or sterexidine or steripod pink or tubulicid or unisept or uriflex c).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
(omeprazole or h 16868 losec or nexium or omesec or prilosec or rapinex or ulcergard or zegerid).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
(ranitidine or ah 19065 or azanplus or biotidin or gr 122311x or pylorid or raciran or raniberl or ranisen or rantec or sostril or taladine or tritec or wal‐zan or zaedoc or zantac).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
(tinidazole or bioshik or fasigin or fasigyne or tindamax or tricolam).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
or/17‐25
-
periodontal therap$.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
oral hygiene.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
(buccal cavity or cavitas oris or mouth or oral cavity or dental hygiene).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
(periodontal and (disease$ or therap$)).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
periodontal scaling.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
periodontal root planing.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
((subgingival or supragingival) adj5 bacterial plaque).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
(periodontal adj10 chemotherapeutic agent$).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
(irrigation adj10 periodontal pocket$).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
gingival pocket.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
or/27‐36
-
11 and 16 and 26 and 37
Appendix 3. EMBASE search strategy
-
Clinical trial.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
Randomized controlled trial.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
Randomization.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
Single‐blind method.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
Double‐blind method.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
Cross‐over studies.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
random allocation.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
placebo.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
randomi?ed controlled trial$.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
Rct.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
Random allocation.tw.
-
randomly allocated.tw.
-
allocated randomly.tw.
-
(allocated adj2 random).tw.
-
single blind$.tw.
-
double blind$.tw.
-
((treble or triple) adj blind$).tw.
-
placebo$.tw.
-
prospective study.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
or/1‐19
-
case study.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
case report.tw.
-
abstract report/ or letter.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
or/21‐23
-
20 not 24
-
H‐pylori.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
H‐pylori infection.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
Helicobacter pylori.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
helicobacter pylori infection.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
or/26‐29
-
PPI.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
proton pump inhibitor$.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
(clarithromycin or 6‐o‐methylerythromycin or abbott‐56268 or biaxin or clarith or klaricid or "te 031").mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
(amoxicillin or actimoxi or almodan or amix or amopen or amoram or amoxicot or amoxidin or amoxil or amoxymed or amrit or biomox or brl 2333 or clamoxyl or dispermox or flemoxin solutab or galenamox or larotid or moxatag or moxilin or p‐hydroxyampicillin or penamox or polymox or respillin or rimoxallin or senox or sumox or trimox or utimox or wymox or zoxycil).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
(chlorhexidine or acclean or acriflex or avagard or betasept or calgon vesta or chloraprep or chlorhexamed or chlorhexigard or chlorohex 2000 or chlorostat or corsodyl or curasept ads 220 or cx antiseptic dusting or denticare or dyna‐hex or eludril or excel or gibitan or habistat or hexidine or hibiclens or hibident or hibiscrub or hibisol or hibitane or mk 412a or novalsan or oris or oro clense or peridex or periochip or periogard or periosep or perisol or phiso‐med or pre‐scrub ii or rotersept or sebidin a or spectrum‐4 or sterexidine or steripod pink or tubulicid or unisept or uriflex c).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
(omeprazole or h 16868 losec or nexium or omesec or prilosec or rapinex or ulcergard or zegerid).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
(ranitidine or ah 19065 or azanplus or biotidin or gr 122311x or pylorid or raciran or raniberl or ranisen or rantec or sostril or taladine or tritec or wal‐zan or zaedoc or zantac).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
(tinidazole or bioshik or fasigin or fasigyne or tindamax or tricolam).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
or/31‐38
-
(periodontal and (therap$ or disease$)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
(oral hygiene or buccal cavity or dental hygiene).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
periodontal scaling.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
oral cavity.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
dental plaque$.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
gingival pocket$.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
periodontal root planing.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
periodontitis.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
preventive dentistry.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
mouth hygiene.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
oral hygiene.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
subgingival bacterial plaque.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
(tooth adj4 (calculus or plaque)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
or/40‐52
-
25 and 30 and 39 and 53

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Eradication therapy combined with periodontal therapy versus eradication therapy alone, Outcome 1 Eradication rate of gastricH.pylori.
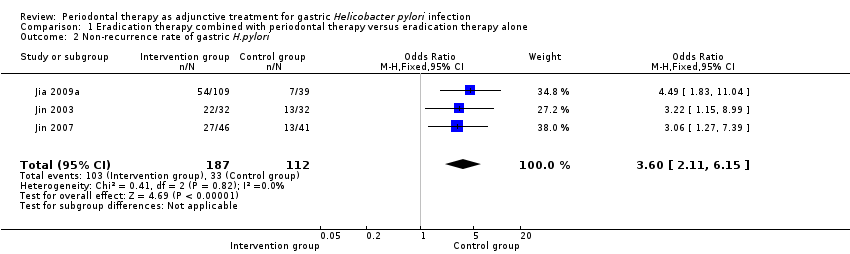
Comparison 1 Eradication therapy combined with periodontal therapy versus eradication therapy alone, Outcome 2 Non‐recurrence rate of gastric H.pylori.

Comparison 2 Subgroup analysis: Stratified by different oral H.pylori status, Outcome 1 Eradication rate of gastricH.pylori.

Comparison 3 Subgroup analysis: Stratified by different durations of periodontal therapy, Outcome 1 Eradication rate of gastricH.pylori.
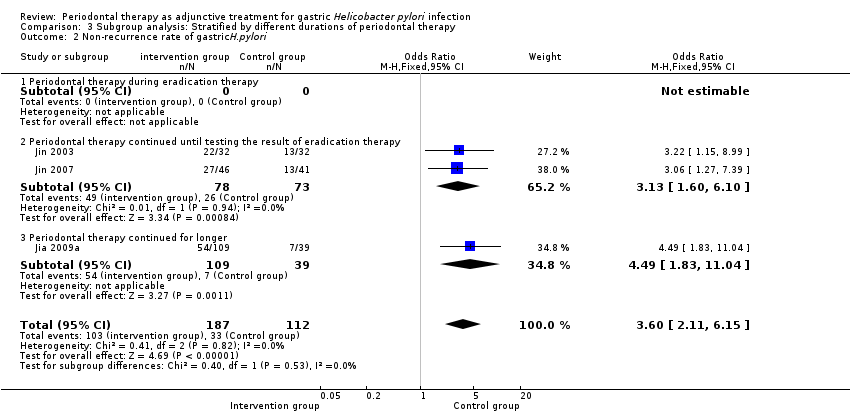
Comparison 3 Subgroup analysis: Stratified by different durations of periodontal therapy, Outcome 2 Non‐recurrence rate of gastricH.pylori.

Comparison 4 Subgroup analysis: After removing low‐quality studies, Outcome 1 Eradication rate of gastric H.pylori.
| Eradication therapy combined with periodontal therapy versus eradication therapy alone for gastric Helicobacter pylori infection | |||||
| Patient or population: patients with gastric Helicobacter pylori infection | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Eradication therapy alone | Eradication therapy combined with periodontal therapy | ||||
| Eradication rate of gastric H. pylori | 60 per 100 | 76 per 100 | OR 2.15 | 543 | ⊕⊕⊕⊝ |
| Non‐recurrence rate of gastric H. pylori | 29 per 100 | 60 per 100 | OR 3.60 | 299 | ⊕⊕⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded one level due to risk of bias (only Zaric 2009 described methods of randomization). | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Eradication rate of gastricH.pylori Show forest plot | 6 | 543 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.15 [1.47, 3.14] |
| 2 Non‐recurrence rate of gastric H.pylori Show forest plot | 3 | 299 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.60 [2.11, 6.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Eradication rate of gastricH.pylori Show forest plot | 6 | 543 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.15 [1.47, 3.14] |
| 1.1 Patients with defined oral H.pylori | 3 | 327 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.42 [1.52, 3.87] |
| 1.2 Patients who were not tested for oral H.pylori | 3 | 216 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.88, 3.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Eradication rate of gastricH.pylori Show forest plot | 6 | 543 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.15 [1.47, 3.14] |
| 1.1 Periodontal therapy during eradication therapy | 2 | 108 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.72 [1.20, 6.14] |
| 1.2 Periodontal therapy continued until testing the result of eradication therapy | 4 | 435 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.02 [1.31, 3.10] |
| 1.3 Periodontal therapy continued for longer | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Non‐recurrence rate of gastricH.pylori Show forest plot | 3 | 299 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.60 [2.11, 6.15] |
| 2.1 Periodontal therapy during eradication therapy | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Periodontal therapy continued until testing the result of eradication therapy | 2 | 151 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.13 [1.60, 6.10] |
| 2.3 Periodontal therapy continued for longer | 1 | 148 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.49 [1.83, 11.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Eradication rate of gastric H.pylori Show forest plot | 2 | 175 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.18 [1.60, 6.33] |


