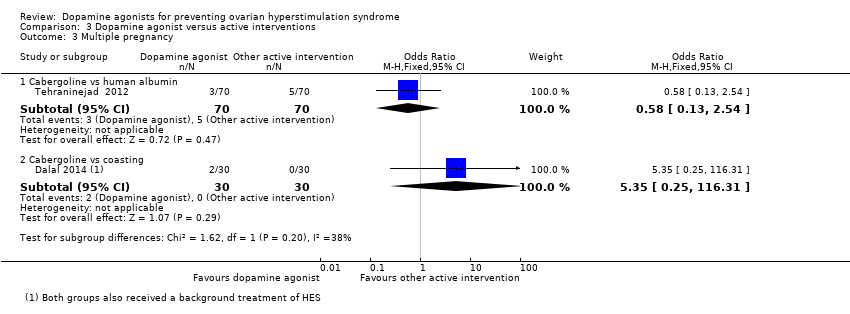
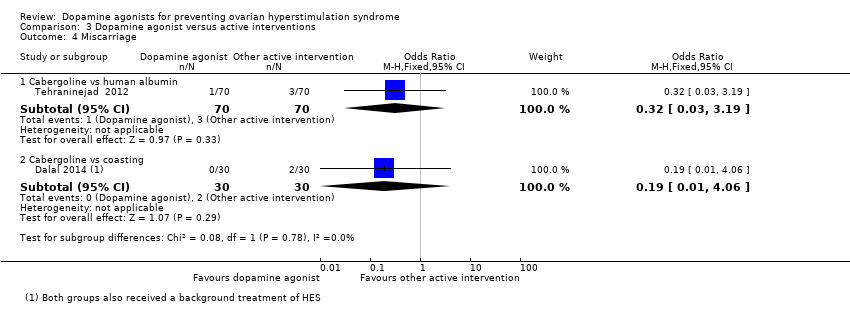
Related content
Related reviews and protocols
Huilin Tang, Selma M. Mourad, Aihua Wang, Suo-Di Zhai, Roger J Hart | 14 April 2021
Arianna D'Angelo, Nazar N Amso, Rudaina Hassan | 23 May 2017
MA Youssef, Selma Mourad | 31 August 2016
Leopoldo O Tso, Michael F Costello, Luiz Eduardo T Albuquerque, Regis B Andriolo, Cristiane R Macedo | 21 December 2020
Mohan S Kamath, Abha Maheshwari, Siladitya Bhattacharya, Kar Yee Lor, Ahmed Gibreel | 2 November 2017
Marian G Showell, Rebecca Mackenzie‐Proctor, Vanessa Jordan, Ruth Hodgson, Cindy Farquhar | 20 December 2018
Arianna D'Angelo, Nazar N Amso | 18 July 2007
Mohamed AFM Youssef, Fulco Van der Veen, Hesham G Al‐Inany, Monique H Mochtar, Georg Griesinger, Mohamed Nagi Mohesen, Ismail Aboulfoutouh, Madelon van Wely | 31 October 2014
Akanksha Sood, Gadha Mohiyiddeen, Gaity Ahmad, Cheryl Fitzgerald, Andrew Watson, Lamiya Mohiyiddeen | 22 November 2021
Mohan S Kamath, Mariano Mascarenhas, Richard Kirubakaran, Siladitya Bhattacharya | 21 August 2020