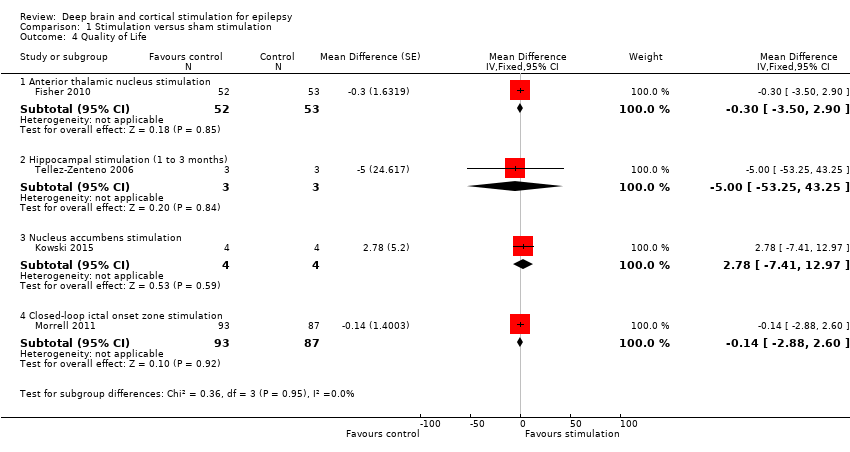
| 1 Seizure freedom RR Show forest plot | 11 | | Risk Ratio (Fixed, 95% CI) | Subtotals only |
|
| 1.1 Anterior thalamic nucleus | 1 | 109 | Risk Ratio (Fixed, 95% CI) | 0.34 [0.01, 8.15] |
| 1.2 Centromedian thalamic stimulation | 1 | 12 | Risk Ratio (Fixed, 95% CI) | 1.0 [0.14, 7.10] |
| 1.3 Cerebellar stimulation | 3 | 33 | Risk Ratio (Fixed, 95% CI) | 0.96 [0.26, 3.52] |
| 1.4 Hippocampal stimulation (1 to 3 months) | 3 | 21 | Risk Ratio (Fixed, 95% CI) | 1.03 [0.25, 4.19] |
| 1.5 Hippocampal stimulation (4 to 6 months) | 1 | 6 | Risk Ratio (Fixed, 95% CI) | 1.67 [0.04, 64.08] |
| 1.6 Nucleus accumbens stimulation | 1 | 8 | Risk Ratio (Fixed, 95% CI) | 1.0 [0.14, 7.10] |
| 1.7 Closed‐loop ictal onset zone stimulation | 1 | 191 | Risk Ratio (Fixed, 95% CI) | 4.85 [0.24, 99.64] |
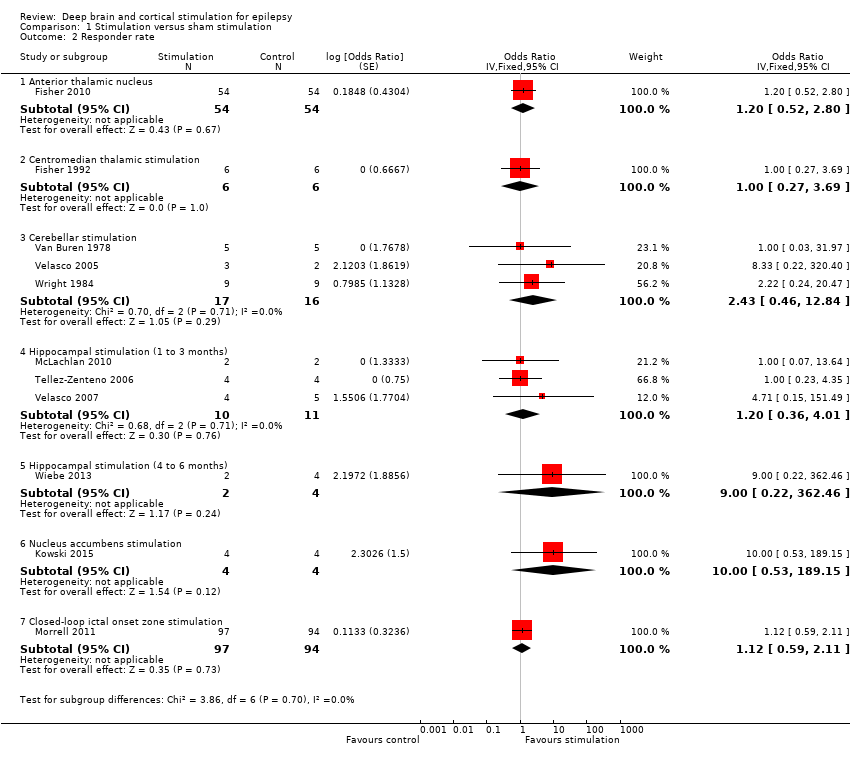
| 2 Responder rate RR Show forest plot | 11 | | Risk Ratio (Fixed, 95% CI) | Subtotals only |
|
| 2.1 Anterior thalamic nucleus | 1 | 108 | Risk Ratio (Fixed, 95% CI) | 1.14 [0.62, 2.10] |
| 2.2 Centromedian thalamic stimulation | 1 | 12 | Risk Ratio (Fixed, 95% CI) | 1.0 [0.38, 2.66] |
| 2.3 Cerebellar stimulation | 3 | 33 | Risk Ratio (Fixed, 95% CI) | 2.00 [0.51, 7.86] |
| 2.4 Hippocampal stimulation (1 to 3 months) | 3 | 21 | Risk Ratio (Fixed, 95% CI) | 1.12 [0.47, 2.66] |
| 2.5 Hippocampal stimulation (4 to 6 months) | 1 | 6 | Risk Ratio (Fixed, 95% CI) | 5.00 [0.29, 87.54] |
| 2.6 Nucleus accumbens stimulation | 1 | 8 | Risk Ratio (Fixed, 95% CI) | 4.00 [0.56, 28.40] |
| 2.7 Closed‐loop ictal onset zone stimulation | 1 | 191 | Risk Ratio (Fixed, 95% CI) | 1.09 [0.69, 1.72] |
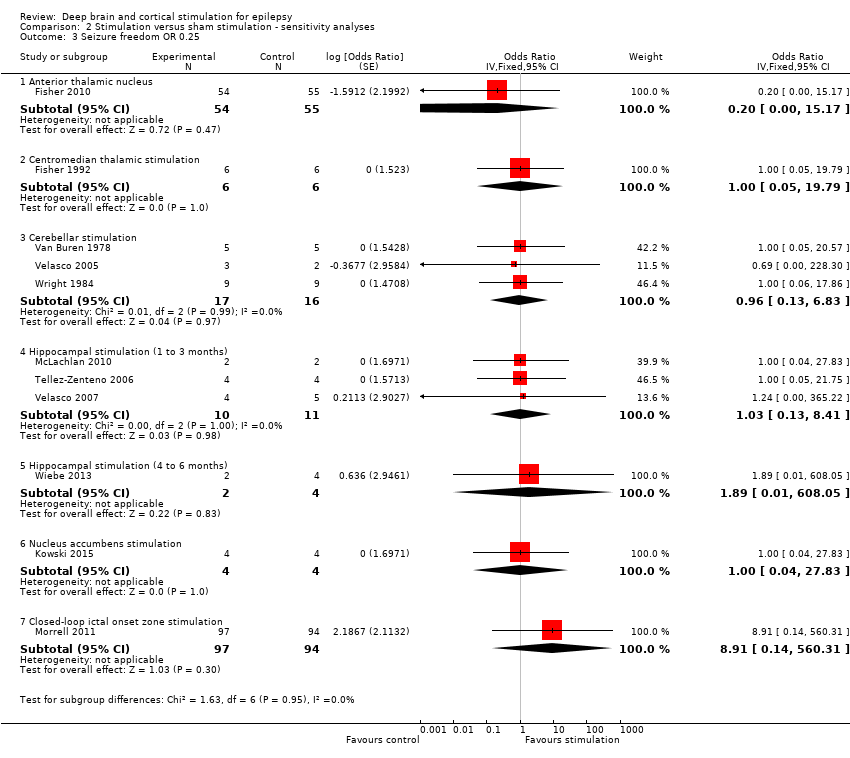
| 3 Seizure freedom OR 0.25 Show forest plot | 11 | | Odds Ratio (Fixed, 95% CI) | Subtotals only |
|
| 3.1 Anterior thalamic nucleus | 1 | 109 | Odds Ratio (Fixed, 95% CI) | 0.20 [0.00, 15.17] |
| 3.2 Centromedian thalamic stimulation | 1 | 12 | Odds Ratio (Fixed, 95% CI) | 1.0 [0.05, 19.79] |
| 3.3 Cerebellar stimulation | 3 | 33 | Odds Ratio (Fixed, 95% CI) | 0.96 [0.13, 6.83] |
| 3.4 Hippocampal stimulation (1 to 3 months) | 3 | 21 | Odds Ratio (Fixed, 95% CI) | 1.03 [0.13, 8.41] |
| 3.5 Hippocampal stimulation (4 to 6 months) | 1 | 6 | Odds Ratio (Fixed, 95% CI) | 1.89 [0.01, 608.05] |
| 3.6 Nucleus accumbens stimulation | 1 | 8 | Odds Ratio (Fixed, 95% CI) | 1.0 [0.04, 27.83] |
| 3.7 Closed‐loop ictal onset zone stimulation | 1 | 191 | Odds Ratio (Fixed, 95% CI) | 8.91 [0.14, 560.31] |
| 4 Responder rate OR 0.25 Show forest plot | 11 | | Odds Ratio (Fixed, 95% CI) | Subtotals only |
|
| 4.1 Anterior thalamic nucleus | 1 | 108 | Odds Ratio (Fixed, 95% CI) | 1.20 [0.52, 2.80] |
| 4.2 Centromedian thalamic stimulation | 1 | 12 | Odds Ratio (Fixed, 95% CI) | 1.0 [0.31, 3.24] |
| 4.3 Cerebellar stimulation | 3 | 33 | Odds Ratio (Fixed, 95% CI) | 2.98 [0.39, 22.77] |
| 4.4 Hippocampal stimulation (1 to 3 months) | 3 | 21 | Odds Ratio (Fixed, 95% CI) | 1.15 [0.35, 3.77] |
| 4.5 Hippocampal stimulation (4 to 6 months) | 1 | 6 | Odds Ratio (Fixed, 95% CI) | 17.00 [0.15, 1934.66] |
| 4.6 Nucleus accumbens stimulation | 1 | 8 | Odds Ratio (Fixed, 95% CI) | 21.00 [0.51, 864.51] |
| 4.7 Closed‐loop ictal onset zone stimulation | 1 | 191 | Odds Ratio (Fixed, 95% CI) | 1.12 [0.59, 2.11] |
| 5 Seizure freedom RR 0.25 Show forest plot | 11 | | Risk Ratio (Fixed, 95% CI) | Subtotals only |
|
| 5.1 Anterior thalamic nucleus | 1 | 109 | Risk Ratio (Fixed, 95% CI) | 0.21 [0.00, 14.95] |
| 5.2 Centromedian thalamic stimulation | 1 | 12 | Risk Ratio (Fixed, 95% CI) | 1.0 [0.06, 15.99] |
| 5.3 Cerebellar stimulation | 3 | 33 | Risk Ratio (Fixed, 95% CI) | 0.96 [0.15, 6.04] |
| 5.4 Hippocampal stimulation (1 to 3 months) | 3 | 21 | Risk Ratio (Fixed, 95% CI) | 1.02 [0.16, 6.46] |
| 5.5 Hippocampal stimulation (4 to 6 months) | 1 | 6 | Risk Ratio (Fixed, 95% CI) | 1.80 [0.01, 369.24] |
| 5.6 Nucleus accumbens stimulation | 1 | 8 | Risk Ratio (Fixed, 95% CI) | 1.0 [0.06, 15.99] |
| 5.7 Closed‐loop ictal onset zone stimulation | 1 | 191 | Risk Ratio (Fixed, 95% CI) | 8.72 [0.14, 538.18] |
| 6 Responder rate RR 0.25 Show forest plot | 11 | | Risk Ratio (Fixed, 95% CI) | Subtotals only |
|
| 6.1 Anterior thalamic nucleus | 1 | 108 | Risk Ratio (Fixed, 95% CI) | 1.14 [0.62, 2.10] |
| 6.2 Centromedian thalamic stimulation | 1 | 12 | Risk Ratio (Fixed, 95% CI) | 1.0 [0.40, 2.52] |
| 6.3 Cerebellar stimulation | 3 | 33 | Risk Ratio (Fixed, 95% CI) | 2.28 [0.40, 13.02] |
| 6.4 Hippocampal stimulation (1 to 3 months) | 3 | 21 | Risk Ratio (Fixed, 95% CI) | 1.08 [0.46, 2.55] |
| 6.5 Hippocampal stimulation (4 to 6 months) | 1 | 6 | Risk Ratio (Fixed, 95% CI) | 9.00 [0.16, 494.41] |
| 6.6 Nucleus accumbens stimulation | 1 | 8 | Risk Ratio (Fixed, 95% CI) | 7.00 [0.44, 111.91] |
| 6.7 Closed‐loop ictal onset zone stimulation | 1 | 191 | Risk Ratio (Fixed, 95% CI) | 1.09 [0.69, 1.72] |


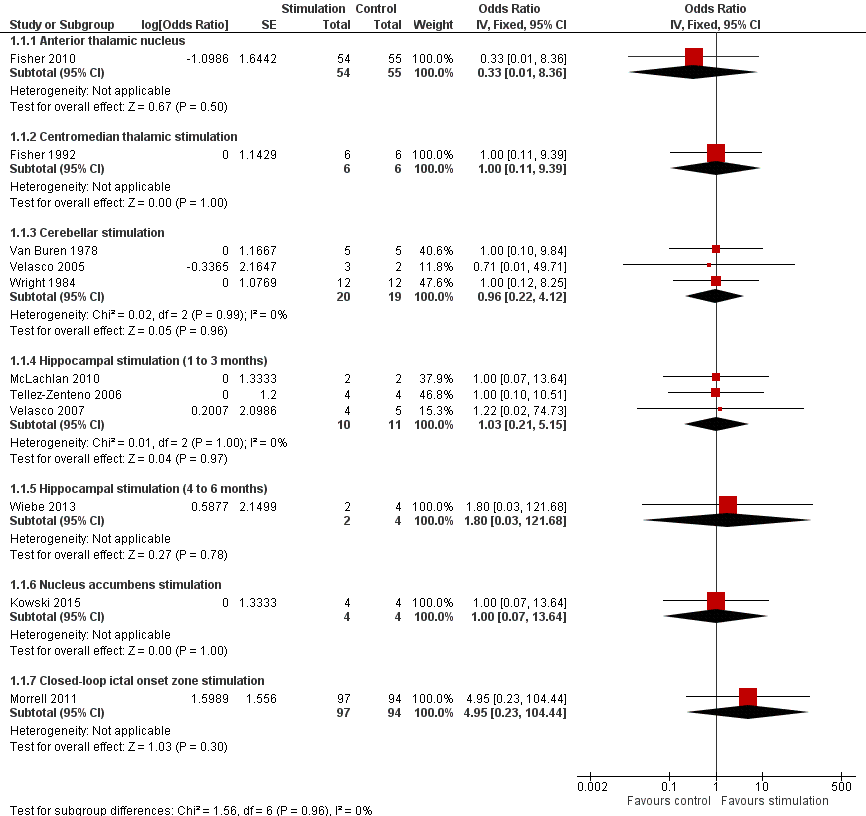

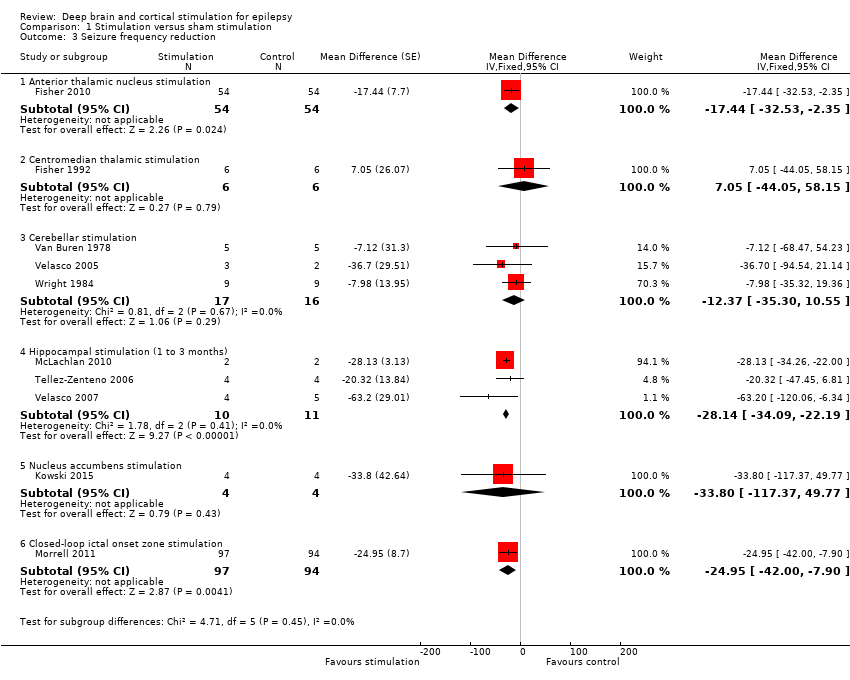
![Forest plot of comparison: 1 Stimulation versus sham stimulation, outcome: 1.3 Seizure frequency reduction.Note: Fisher 2010 (anterior thalamic nucleus stimulation) and Morrell 2011 (closed‐loop ictal onset zone stimulation) estimated the treatment effect and its standard error on a logarithmic scale, using the generalized estimating equation (GEE) model. As in this figure standard errors could not be inputted on the logarithmic scale, the values for the 95% confidence interval presented here differ slightly from the (more correct) values mentioned in the text. These correct values are ‐17.4% with 95% CI [‐31.2;‐1.0] for Fisher 2010 and ‐24.9% with 95% CI [‐40.1;‐6.0] for Morrell 2011.](/cdsr/doi/10.1002/14651858.CD008497.pub3/media/CDSR/CD008497/image_n/nCD008497-AFig-FIG05.png)