Daclizumab para la esclerosis múltiple recurrente remitente
Information
- DOI:
- https://doi.org/10.1002/14651858.CD008127.pub4Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 23 December 2013see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Multiple Sclerosis and Rare Diseases of the CNS Group
- Copyright:
-
- Copyright © 2013 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
Liu J and Wang L formulated the idea and developed the basis for the review.
Zhan S and Xia Y supervised the methodology and statistics.
Liu J and Wang L were in charge of updating the review.
Sources of support
Internal sources
-
Department of Geriatric Neurology, Chinese PLA General Hospital, China.
External sources
-
No sources of support supplied
Declarations of interest
None known.
Acknowledgements
The authors would like to acknowledge the help provided by Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Group.
Version history
| Published | Title | Stage | Authors | Version |
| 2013 Dec 23 | Daclizumab for relapsing remitting multiple sclerosis | Review | Jia Liu, Lu‐Ning Wang, Siyan Zhan, Yinyin Xia | |
| 2012 Apr 18 | Daclizumab for relapsing remitting multiple sclerosis | Review | Jia Liu, Luning Wang, Si‐Yan Zhan, Yinyin Xia | |
| 2010 Jun 16 | Daclizumab for relapsing remitting multiple sclerosis | Review | Jia Liu, Luning Wang, Siyan Zhan, Jiping Tan, Yinyin Xia | |
| 2009 Oct 07 | Daclizumab for relapsing remitting multiple sclerosis | Protocol | Jia Liu, Luning Wang, Siyan Zhan, Jiping Tan, Yinyin Xia | |
Differences between protocol and review
None.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICOs

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
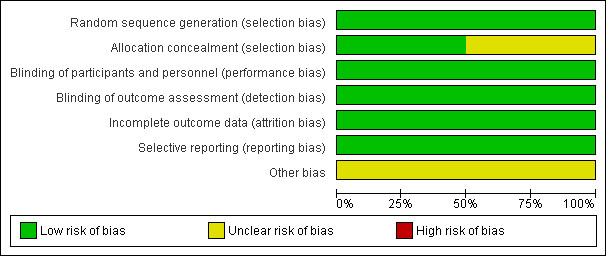
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Safety, Outcome 1 Any adverse event.

Comparison 1 Safety, Outcome 2 Serious adverse event.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any adverse event Show forest plot | 2 | 851 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.89, 1.07] |
| 2 Serious adverse event Show forest plot | 2 | 851 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.29, 4.54] |


