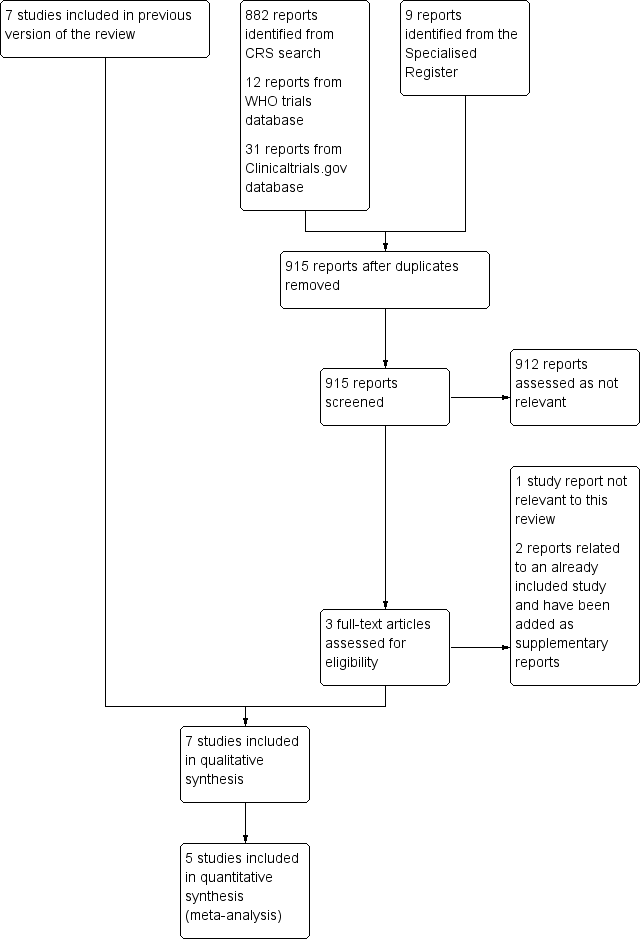
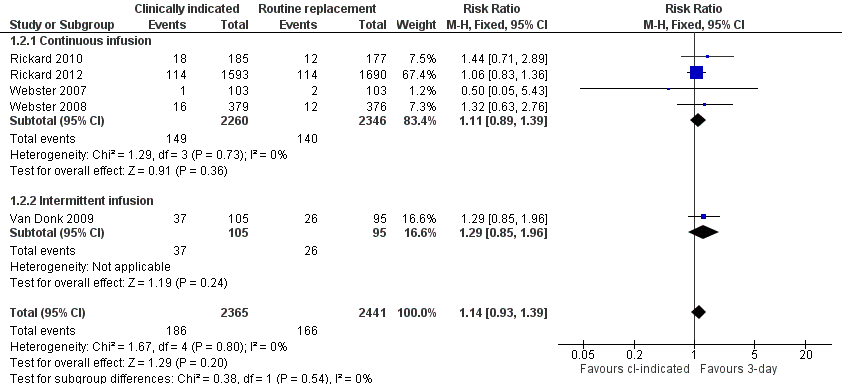
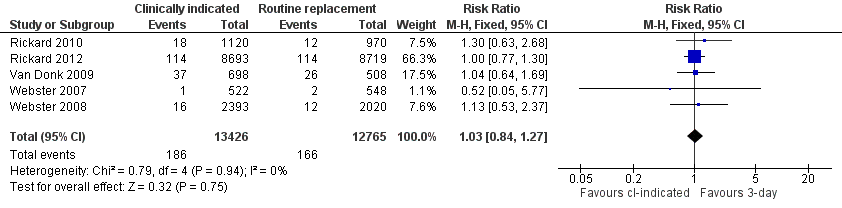
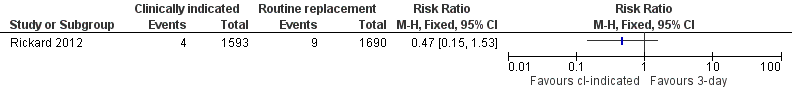
Related content
Related reviews and protocols
Joan Webster, Sonya Osborne, Claire M Rickard, Nicole Marsh | 23 January 2019
Chakrapani Vasudevan, Sam J Oddie, William McGuire | 20 April 2016
Natalie K Bradford, Rachel M Edwards, Raymond J Chan | 30 April 2020
Eduardo López-Briz, Vicente Ruiz Garcia, Juan B Cabello, Sylvia Bort-Martí, Rafael Carbonell Sanchis | 18 July 2022
Prakeshkumar S Shah, Niketa Shah | 25 February 2014
Awaiss Ellahi, Fiona Stewart, Emily A Kidd, Rhonda Griffiths, Ritin Fernandez, Muhammad Imran Omar | 29 June 2021
Guo Hua Zheng, Liu Yang, Hai Ying Chen, Jian Feng Chu, Lijuan Mei | 4 June 2014
Charlie C‐T Hsu, Gigi NC Kwan, Hannah Evans‐Barns, John A Rophael, Mieke L van Driel | 21 August 2016
Marie-Claude Pelland-Marcotte, Nour Amiri, Maria L Avila, Leonardo R Brandão | 18 June 2020
Htay Htay, David W Johnson, Jonathan C Craig, Francesco Paolo Schena, Giovanni FM Strippoli, Allison Tong, Yeoungjee Cho | 31 May 2019
Cochrane Clinical Answers
Jane Burch, Dane Gruenebaum | 22 March 2017