Tratamiento con células madres para el infarto de miocardio agudo
Information
- DOI:
- https://doi.org/10.1002/14651858.CD006536.pub4Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 30 September 2015see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Heart Group
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
Sheila Fisher: methodological expert, eligibility screening, data extraction, quality assessment, data analysis and preparation of the final report.
Huajun Zhang: eligibility screening, data extraction and comment on the final report.
Carolyn Doree: design and implementation of search strategies, initial eligibility screening, data verification and comment on the final report.
Anthony Mathur: clinical content expert, preparation of the final report.
Enca Martin‐Rendon: scientific content expert, eligibility screening, data extraction, quality assessment and preparation of the final report. Corresponding author who takes global responsibility for this review.
Sources of support
Internal sources
-
NHS Blood and Transplant, Research and Development (CD and SW), UK.
-
William Harvey Research Institute (AM), UK.
External sources
-
National Institute of Health Research (NIHR), UK.
This work was supported by NIHR under its Cochrane Incentive Award scheme (award number 14/175/34 to EMR). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Declarations of interest
Sheila Fisher: none known.
Huajun Zhang: none known.
Carolyn Doree: none known.
Anthony Mathur: none known.
Enca Martin‐Rendon: none known.
Acknowledgements
We acknowledge with thanks the contributions of Susan Brunskill, Dr David Clifford, Professor C. Hyde, Dr Simon Stanworth and Professor Suzanne Watt, for their contribution to previous versions of this review. We thank Pat Tsang for the translation of Xiao 2012 from Mandarin to English for this update and Mrs F‐J Lu for translating papers from Mandarin to English in the original version of this review.
We are grateful to those trial investigators who kindly responded to our questions and requests for further clarification or information on published and unpublished studies in this update of the review: Dr B Assmus and Prof. V Schachinger (JW Goethe University, Frankfurt, Germany), Prof. M Tendera and Prof. W Wojakowski (Medical University of Silesia, Poland), Prof. S Grajek (Poznan University of Medical Science, Poland), Prof. L Chojnowska (Institute of Cardiology, Warsaw, Poland), Dr K Lunde (Oslo University Hospital, Norway), Prof. M Plewka and Dr P Lipiec (Medical University of Lodz, Poland), Prof. V Ryabov (FBGU Institute of Cardiology SB RAMS, Russia), Prof. A Hirsch (University of Amsterdam, The Netherlands), Prof. RG Turan (University Hospital Cologne, Germany), Prof. P Lemarchand (University of Nantes, France), Prof. J Roncalli (Hôpital Ranguei, Toulouse, France) and Dr M Piepoli (Guglielmo da Saliceto Policherurgico Hospital, Italy).
Version history
| Published | Title | Stage | Authors | Version |
| 2015 Sep 30 | Stem cell treatment for acute myocardial infarction | Review | Sheila A Fisher, Huajun Zhang, Carolyn Doree, Anthony Mathur, Enca Martin‐Rendon | |
| 2012 Feb 15 | Stem cell treatment for acute myocardial infarction | Review | David M Clifford, Sheila A Fisher, Susan J Brunskill, Carolyn Doree, Anthony Mathur, Suzanne Watt, Enca Martin‐Rendon | |
| 2008 Oct 08 | Stem cell treatment for acute myocardial infarction | Review | Enca Martin‐Rendon, Susan Brunskill, Carolyn Doree, Chris Hyde, Anthony Mathur, Simon Stanworth, Suzanne Watt | |
| 2007 Apr 18 | Stem cell treatment for acute myocardial infarction | Protocol | Enca Martin‐Rendon, Susan Brunskill, Carolyn J Doree, Chris Hyde, Suzanne Watt, Anthony Mathur | |
Differences between protocol and review
The original outcomes of this review have been revised in this update, focusing on clinical outcomes. However, the surrogate endpoint of LVEF is a standard, widely reported marker for cardiac function and has been retained as a reference point with other trials and systematic reviews in AMI. Surrogate outcomes other than LVEF reported in previous versions of this review, namely engraftment and survival of the infused stem cells, left ventricular end‐systolic volume, left ventricular end‐diastolic volume, wall motion score, stroke volume index and infarct size are no longer included. We now define revised primary outcomes as (i) all‐cause mortality, (ii) cardiovascular mortality, (iii) composite measures of major adverse cardiac events (MACE), and (iv) periprocedural adverse events. Secondary outcomes include morbidity, LVEF and quality of life and performance measures.
In the protocol and previous versions of the review we implemented fixed‐effect models in the first instance. It is now clear that there are many potential sources of heterogeneity across trials, and in this version of the review we have performed meta‐analyses using random‐effects models throughout.
In the writing of this version of the review we identified a systematic error in the previous versions of the review in the calculation of standard deviations for mean change from baseline LVEF values. This issue has now been corrected. In some studies it was not possible to accurately calculate the value of the standard deviation. These studies, previously analysed as mean change from baseline values, are now reported as mean value at endpoint; results from combined analyses of mean change from baseline and endpoint values are reported.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICOs
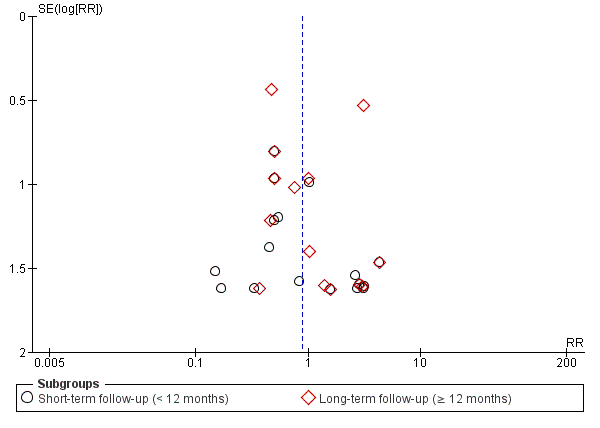
Funnel plot of comparison: 1 Cells compared to no cells, outcome: 1.1 All‐cause mortality.

Trial sequential analysis of all‐cause mortality at long term follow‐up, assuming a long‐term mortality incidence rate of 6.1% in controls and a relative risk reduction of 35% in cell therapy patients
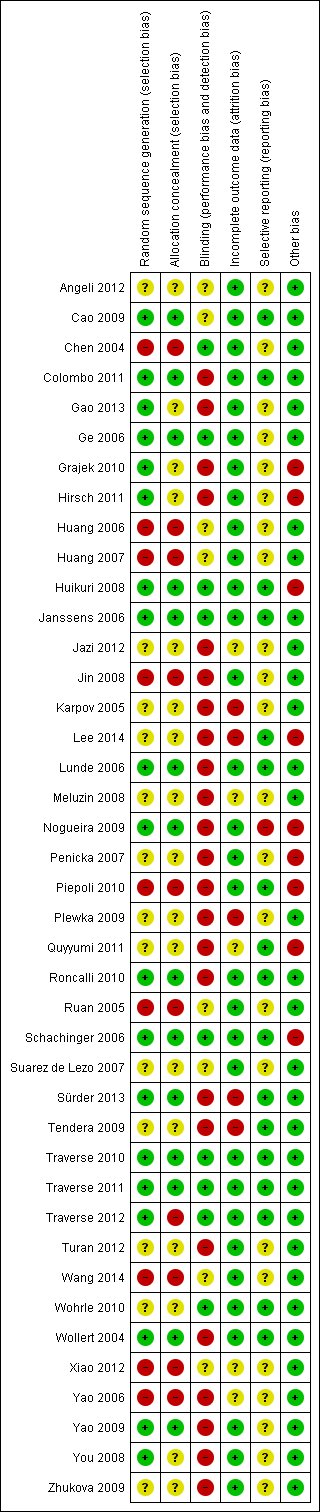
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Cells compared to no cells, Outcome 1 All‐cause mortality.

Comparison 1 Cells compared to no cells, Outcome 2 Cardiovascular mortality.

Comparison 1 Cells compared to no cells, Outcome 3 Composite measure of death, reinfarction, re‐hospitalisation for heart failure.

Comparison 1 Cells compared to no cells, Outcome 4 Incidence of reinfarction.

Comparison 1 Cells compared to no cells, Outcome 5 Incidence of re‐hospitalisation for heart failure.

Comparison 1 Cells compared to no cells, Outcome 6 Incidence of target vessel revascularisation.

Comparison 1 Cells compared to no cells, Outcome 7 Incidence of arrhythmias.

Comparison 1 Cells compared to no cells, Outcome 8 Incidence of restenosis.

Comparison 1 Cells compared to no cells, Outcome 9 Quality of life measures.

Comparison 1 Cells compared to no cells, Outcome 10 NYHA classification.

Comparison 1 Cells compared to no cells, Outcome 11 Exercise tolerance.

Comparison 1 Cells compared to no cells, Outcome 12 Maximum VO2 (mL/kg/min).

Comparison 1 Cells compared to no cells, Outcome 13 VE/VCO2 slope.

Comparison 1 Cells compared to no cells, Outcome 14 Peak heart rate (bpm).

Comparison 1 Cells compared to no cells, Outcome 15 LVEF measured by MRI (<12 months).

Comparison 1 Cells compared to no cells, Outcome 16 LVEF measured by MRI (≥ 12 months).

Comparison 1 Cells compared to no cells, Outcome 17 LVEF measured by echocardiography (< 12 months).

Comparison 1 Cells compared to no cells, Outcome 18 LVEF measured by echocardiography (≥12 months).

Comparison 1 Cells compared to no cells, Outcome 19 LVEF measured by SPECT (< 12 months).

Comparison 1 Cells compared to no cells, Outcome 20 LVEF measured by SPECT (≥ 12 months).

Comparison 1 Cells compared to no cells, Outcome 21 LVEF measured by left ventricular angiography (< 12 months).

Comparison 1 Cells compared to no cells, Outcome 22 LVEF measured by left ventricular angiography (≥ 12 months).

Comparison 1 Cells compared to no cells, Outcome 23 LVEF measured by radionuclide ventriculography (RNV) (<12 months).

Comparison 1 Cells compared to no cells, Outcome 24 LVEF measured by radionuclide ventriculography (≥ 12 months).

Comparison 2 Sensitivity analysis ‐ route of cell delivery, Outcome 1 All‐cause mortality (< 12 months).

Comparison 3 Sensitivity analysis ‐ selection bias, Outcome 1 All‐cause mortality (< 12 months).

Comparison 4 Sensitivity analysis ‐ attrition bias, Outcome 1 All‐cause mortality (< 12 months).

Comparison 4 Sensitivity analysis ‐ attrition bias, Outcome 2 All‐cause mortality (≥ 12 months).

Comparison 4 Sensitivity analysis ‐ attrition bias, Outcome 3 Cardiovascular mortality (< 12 months).

Comparison 4 Sensitivity analysis ‐ attrition bias, Outcome 4 Cardiovascular mortality (≥ 12 months).

Comparison 5 Sensitivity analysis ‐ performance bias, Outcome 1 All‐cause mortality (< 12 months).

Comparison 5 Sensitivity analysis ‐ performance bias, Outcome 2 All‐cause mortality (≥ 12 months).

Comparison 6 Subgroup analysis ‐ baseline LVEF measured by MRI, Outcome 1 All‐cause mortality (< 12 months).

Comparison 6 Subgroup analysis ‐ baseline LVEF measured by MRI, Outcome 2 All‐cause mortality (≥ 12 months).

Comparison 6 Subgroup analysis ‐ baseline LVEF measured by MRI, Outcome 3 LVEF measured by MRI (< 12 months).

Comparison 6 Subgroup analysis ‐ baseline LVEF measured by MRI, Outcome 4 LVEF measured by MRI (≥ 12 months).

Comparison 7 Subgroup analysis ‐ cell type, Outcome 1 All‐cause mortality (< 12 months).

Comparison 7 Subgroup analysis ‐ cell type, Outcome 2 All‐cause mortality (≥ 12 months).

Comparison 8 Subgroup analysis ‐ dose of stem cells, Outcome 1 All‐cause mortality (< 12 months).

Comparison 8 Subgroup analysis ‐ dose of stem cells, Outcome 2 All‐cause mortality (≥ 12 months).

Comparison 8 Subgroup analysis ‐ dose of stem cells, Outcome 3 LVEF measured by MRI (< 12 months).

Comparison 8 Subgroup analysis ‐ dose of stem cells, Outcome 4 LVEF measured by MRI (≥ 12 months).

Comparison 8 Subgroup analysis ‐ dose of stem cells, Outcome 5 LVEF measured by left ventricular angiography (< 12 months).

Comparison 9 Subgroup analysis ‐ timing of cell administration, Outcome 1 All‐cause mortality (< 12 months).

Comparison 9 Subgroup analysis ‐ timing of cell administration, Outcome 2 All‐cause mortality (≥ 12 months).

Comparison 9 Subgroup analysis ‐ timing of cell administration, Outcome 3 LVEF measured by MRI (< 12 months).

Comparison 9 Subgroup analysis ‐ timing of cell administration, Outcome 4 LVEF measured by MRI (≥ 12 months).

Comparison 9 Subgroup analysis ‐ timing of cell administration, Outcome 5 LVEF measured by left ventricular angiography (< 12 months).

Comparison 10 Subgroup analysis ‐ heparinised cell solution, Outcome 1 All‐cause mortality (< 12 months).

Comparison 10 Subgroup analysis ‐ heparinised cell solution, Outcome 2 All‐cause mortality (≥ 12 months).

Comparison 10 Subgroup analysis ‐ heparinised cell solution, Outcome 3 LVEF measured by MRI (< 12 months).

Comparison 10 Subgroup analysis ‐ heparinised cell solution, Outcome 4 LVEF measured by MRI (≥ 12 months).

Comparison 10 Subgroup analysis ‐ heparinised cell solution, Outcome 5 LVEF measured by left ventricular angiography (< 12 months).
| Cells compared to no cells for acute myocardial infarction (AMI) | ||||||
| Patient or population: patients with AMI | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No cells | Cells | |||||
| All‐cause mortality ‐ short‐term follow‐up (< 12 months) | Study population | RR 0.80 | 1365 | ⊕⊕⊕⊝ | Further research may change the estimate | |
| 28 per 1000 | 23 per 1000 | |||||
| All‐cause mortality ‐ long‐term follow‐up (≥ 12 months) | Study population | RR 0.93 | 996 | ⊕⊕⊕⊝ | Further research may change the estimate | |
| 70 per 1000 | 65 per 1000 | |||||
| Cardiovascular mortality ‐ short‐term follow‐up (< 12 months) | Study population | RR 0.72 | 290 | ⊕⊕⊕⊝ | Further research may change the estimate | |
| 54 per 1000 | 39 per 1000 | |||||
| Cardiovascular mortality ‐ long‐term follow‐up (≥ 12 months) | Study population | RR 1.04 | 527 | ⊕⊕⊕⊝ | Further research may change the estimate | |
| 72 per 1000 | 75 per 1000 | |||||
| Composite death, reinfarction and hospitalisation for heart failure ‐ short‐term follow‐up (< 12 months) | Study population | RR 0.36 | 379 | ⊕⊕⊕⊝ | Further research may change the estimate | |
| 66 per 1000 | 24 per 1000 | |||||
| Composite death, reinfarction and hospitalisation for heart failure ‐ long‐term follow‐up (≥ 12 months) | Study population | RR 0.63 | 497 | ⊕⊕⊕⊝ | Further research may change the estimate | |
| 140 per 1000 | 88 per 1000 | |||||
| *The assumed risk is based on the observed incidence across the pooled control groups. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Imprecision: information size criterion not met. Small size effect. | ||||||
| Study ID | Country of study | Patient population | Mean (SD) age of participants (years) | % Male | No. randomised participants receiving intervention | No. randomised participants receiving comparator | Mean duration of follow‐up |
| Brazil | STEMI with LVEF < 45%; successful PCI | n/r | n/r | 11 | 11 | 12 months | |
| China | STEMI; PCI within 12 hours, often with drug‐eluting stent implantation | BMMNC: 50.7 (SEM 1.1) | BMMNC: 95.1% | 41 | 45 | 48 months | |
| China | AMI; PCI within 12 hours, mostly with stent implantation | BMMNC: 58 (7.0) | BMMNC: 94% | 34 | 35 | 6 months | |
| Italy | Large anterior STEMI; PCI with bare metal stent implantation within 12 hours | CD133+: median 54 (range 47 to 60) | CD133+: 100% | 5 | 5 | 12 months | |
| China | Acute STEMI; PCI with stent implantation within 12 hours | BM‐MSC: 55.0 (SEM 1.6) | BM‐MSC: 100% | 21 | 22 | 24 months | |
| China | First STEMI within 24 hours; PCI with stent implantation | BMMNC: 58 (11) | BMMNC: 80% | 10 | 10 | 6 months | |
| Poland | First anterior AMI; PCI within 12 hours with bare metal stent implantation | BMMNC: 49.9 (8.4) | BMMNC: 87% | 31 | 14 | 12 months | |
| Hirsch 2011 | The Netherlands | First STEMI; PCI with stent implantation within 12 hours | BMMNC: 56 (9) | BMMNC: 84% | 69 | 65 | 60 months |
| China | AMI; PCI within 24 hours | BMMNC: 57.3 (10.1) | BMMNC: 65% | 20 | 20 | 6 months | |
| China | AMI; PCI within 24 hours with bare metal (35%) or drug‐eluting (65%) stent implantation | BMMNC: 54.8 (5.8) | BMMNC: 85% | 20 | 20 | 6 months | |
| Huikuri 2008 | Finland | STEMI; thrombolytic drugs initiated within 12 hours | BMMNC: 60 (10) | BMMNC: 90% | 40 | 40 | 6 months |
| Belgium | STEMI; PCI with bare metal stent implantation at median 3.7 hours (IQR 2.5 to 7.6) | BMMNC: 55.8 (11) | BMMNC: 82% | 33 | 34 | 4 months | |
| Iran | Anterior MI within 1 month with a history of anterior MI and LVEF < 35%; PCI | BMMNC: 48.0 (SEM 2.5) | BMMNC: 66% | n/r | n/r | 6 months | |
| China | AMI; thrombolytic drugs and PCI | BMMNC: 62.3 (7.7) | BMMNC: 71.4% | 14 | 12 | 12 months | |
| Russia | STEMI; PCI with bare metal stent implantation within 6.6 (4.9) hours and thrombolytic drugs | BMMNC: 55.2 (8.6) | BMMNC: 90% | 28 | 34 | 8.2 (0.72) years | |
| Lee 2014 | South Korea | STEMI within 24 hours enrolled < 72 hours after revascularisation by | BM‐MSC: 53.9 (10.5) | BM‐MSC: 90.0% | 40 | 40 | 6 months |
| Lunde 2006 | Norway | Anterior STEMI; PCI within 2 to 24 hours | BMMNC: 58.1 (8.5) | BMMNC: 84% | 50 | 51 | 36 months |
| Czech Republic | First STEMI; PCI with stent implantation within 12 hours or 3 days | BMMNC: 54 (SEM 2) | BMMNC: 90% (HD), 95% (LD) | n/r (a) | n/r (a) | 12 months | |
| Nogueira 2009 | Brazil | STEMI; thrombolytic drugs and PCI with stent implantation within 24 hours | BMMNC: 59.7 (14.3) (AG), 53.6 (8.3) (VG) | BMMNC: 71% (AG), 70% (VG) | 24 (14 AG, 10 VG) | 6 | 6 months |
| Czech Republic | First anterior STEMI and LVEF ≤ 50% | BMMNC: 61 (14) | BMMNC: 71% | 17 | 10 | 24 months | |
| Piepoli 2010 | Italy | Anterior STEMI; PCI with stent implantation within 2 to 6 hours | BMMNC: 63.1 (SEM 2.7) | BMMNC: 68.4% | 19 | 19 | 24 months |
| Poland | First anterior STEMI and LVEF < 40%; PCI within 12 hours | BMMNC: 59 (9) | BMMNC: 68% | 40 | 20 | 24 months | |
| Quyyumi 2011 | USA | Acute STEMI and LVEF ≤ 50% | CD34+: median 50.5 (IQR 45 ‐ 53) (HD), 63.0 (IQR 57 ‐ 66) (MD), 52.0 (IQR 51 ‐ 52) (LD) | CD34+: 100% (HD), 80% (MD), 80% (LD) | 16 (5 LD, 5 MD, 6 HD) | 15 | 12 months |
| Roncalli 2010 | France | Acute STEMI and LVEF ≤ 45%; PCI with bare metal stent implantation within 24 hours | BMMNC: 56 (12) | BMMNC: 80.8% | 52 | 49 | 12 months |
| China | AMI admitted within mean 12.1 (12.6) hours of onset; PCI | BMMNC: 61 (8) | BMMNC: 88.9 | 9 | 11 | 6 months | |
| Schachinger 2006 | Germany; Switzerland | Acute STEMI and visual estimated LVEF ≤ 45%; PCI with stent implantation at mean 7.5 (8.0) hours | BMMNC: 55 (11) | BMMNC: 82% | 101 | 103 | 60 months |
| Spain | Anterior STEMI within 12 hours; PCI (some with stent) or thrombolytics | BMMNC: 52 (12) | BMMNC: 80% | 10 | 10 | 3 months | |
| Sürder 2013 | Switzerland | Large STEMI with LVEF < 45%; thrombolytics and PCI with stent within 24 hours | BMMNC: median 55 (IQR 15) (E), 62 (IQR 15) (L) | BMMNC: 86.2% (E), 82.5 (L) | 133 (66 E, 67 L) | 67 | 12 months |
| Tendera 2009 | Poland | Anterior AMI and LVEF ≤ 40% | CD34/CXCR4+: median 58 BMMNC: median 55 | CD34/CXCR4+: 63.7% BMMNC: 70.6% | 160 (80 CD34/CXCR4+, 80 BMMNC) | 40 | 6 months |
| USA | First anterior STEMI; PCI mostly with drug‐eluting stent implantation | BMMNC: median 52.5 (IQR 43 ‐ 64) | BMMNC: 83.3% | 30 | 10 | 15 months | |
| Traverse 2011 | USA | STEMI with LVEF ≤ 45%; PCI with stent, mostly drug‐eluting, at median 3.4 (IQR 2.3 to 14.3) hours | BMMNC: 57.6 (11) | BMMNC: 79% | 59 | 29 | 6 months |
| Traverse 2012 | USA | Anterior STEMI with LVEF < 45%; PCI with stent, mostly drug‐eluting | BMMNC: 55.6 (10.8) (day 3)/58.2 (11.3) day 7) | BMMNC: 88.4% (day 3)/86.1% (day 7) | 43 (day 3) | 24 (day 3) | 12 months |
| Germany | Acute STEMI; PCI with stent implantation | BMMNC: 61 (15) | BMMNC: 67% | 42 | 20 | 12 months | |
| China | Acute STEMI; PCI predominantly with stent implantation within 8 hours | BM‐MSC: 58 (10.2) | BM‐MSC: 67.9% | 30 | 30 | 6 months | |
| Wohrle 2010 | Germany | AMI; PCI with stent, some drug eluting, within 6 to 48 hours | BMMNC: 61.0 (8.1) | BMMNC: 90% | 29 | 13 | 36 months |
| Wollert 2004 | Germany | STEMI within 5 days; PCI with bare metal stent implantation, some with thrombolytic drugs | BMMNC: 53.4 (14.8) | BMMNC: 67% | 33 | 32 | 60 months |
| China | AMI; undergoing elective PCI within 4 weeks of AMI | BM‐MSC: 60.4 (8.9) | BM‐MSC: 58.8% | 17 | 21 | 3 months | |
| China | STEMI within 1 week; PCI | BMMNC: 58.3 (9.5) | BMMNC: 89.1% | 92 | 92 | 30 months | |
| China | First anterior STEMI; PCI within 12 hours | BMMNC: 52.1 (6.3) (SD), 51.3 (7.4) (DD) | BMMNC: 83.3& (SD), 80.0% (DD) | 30 (15 SD, 15 DD) | 15 | 12 months | |
| China | AMI within 24 hours; thombolytic reperfusion | BM‐MSC: 60.5 | BM‐MSC: 71.4% | 7 | 16 | 8 weeks | |
| Russia | MI of the front wall; thrombolytic drugs and/or PCI with stent implantation | BMMNC: 48 (7) | BMMNC: 100% | 8 | 3 | 36 months | |
| STEMI, ST‐segment elevation myocardial infarction; AMI, acute myocardial infarction; PCI, percutaneous coronary intervention; LVEF, left ventricular ejection fraction; BMMNC, bone marrow mononuclear cells; BM‐MSC, bone marrow mesenchymal stem cells; SEM, standard error of the mean; SD, standard deviation; LD, low dose; MD, moderate dose; HD, high dose; AG, arterial group; VG, venous group; E, early cells; L, late cells; S, selected cells; U, unselected cells; SD, single dose; DD, double dose (a)Meluzin 2008: 73 participants were randomised in total ‐ the number randomised to each group was not reported. | |||||||
| Study ID | Time of cell administration | Intervention given by: | Route of cell administration | Intervention cell type | How are cells obtained? (*) | What were they re‐suspended in? | Dose administered? | Comparator arm (placebo or control) |
| 5 to 9 days after AMI | Cardiologist | Infusion into IRCA | BMMNC | n/r | n/r | 260 (160) million cells | Placebo (n/r) | |
| 7 days after PCI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Heparinised saline | 500 million cells | Placebo (heparinised saline) | |
| Mean 18.4 (0.5) days after PCI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Heparinised saline | 48,000 (60,000) million cells | Placebo (heparinised saline) | |
| Day 9 to 16 after PCI | Cardiologist | Infusion into IRCA | CD133‐positive cells | BM aspiration (**), immunomagnetic selection to isolate CD133‐positive cells | 0.9% saline solution and 10% human serum albumin | Median (range): 5.9 (4.9 to 13.5) million cells | No additional therapy (Control) | |
| Mean 17.1 (0.6) hours after PCI | Cardiologist | Infusion into IRCA | BM‐MSC | BM aspiration (**), culture for 14 days to select MSC | Heparinised saline | 3.08 (0.52) million cells | No additional therapy (Control) | |
| Within 15 hours of AMI | Cardiologist | Infusion into IRCA | BMMNC | n/r | n/r | 40 million cells | Placebo (n/r) | |
| 5 to 6 days after PCI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | X‐vivo 15 medium and 2% autologous plasma | 410 (180) million cells | No additional therapy (Control) | |
| Hirsch 2011 | 3 to 8 days after PCI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Heparinised saline and 4 % human serum albumin | 296 (164) million cells | No additional therapy (Control) |
| Within 2 hours of PCI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Heparinised saline | 180 (420) million cells | Placebo (heparinised saline) | |
| Within 2 hours of PCI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Heparinised saline | 120 (650) million cells | Placebo (heparinised saline) | |
| Huikuri 2008 | Mean 70 (36) hours after thombolysis | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Heparinised saline and 50% autologous serum | 402 (196) million cells | Placebo (heparinised saline and 50% autologous serum) |
| Within 20 hours of PCI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Heparinised saline and 5% autologous serum solution | 172 (72) million cells | Placebo (heparinised saline and 5% autologous serum) | |
| Within 1 month of AMI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | M199 medium containing VEGF, bFGF, IGF‐1 and 10% human serum | 2460 (SEM 840) million cells | No additional therapy (Control) | |
| At least 7 to 10 days after AMI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Heparinised saline | 62.7 (17.5) million cells | No additional therapy (Control) | |
| 7 to 21 days after AMI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | n/r | 88.5 (49.2) million cells | No additional therapy (Control) | |
| Lee 2014 | 25 (2.4) days after BM aspiration at 3.8 (1.5) days after admission | Cardiologist | Infusion into IRCA | BM‐MSC | BM aspiration (**), culture for 2 to 3 weeks to isolate MSC | n/r | 72 (9) million cells | No additional therapy (Control) |
| Lunde 2006 | 4 to 8 days after AMI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Heparinised plasma | Median (interquartile range): 68 (54 to 130) million cells | No additional therapy (Control) |
| 5 to 9 days (mean 7 (0.3) days) after AMI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | n/r | LD: 10 million cells (range: 9 to 20 million) HD: 100 million cells (90 to 200 million cells) | No additional therapy (Control) | |
| Nogueira 2009 | AG: 3 to 6 days (mean 5.5 (1.28) days) after PCI VG: 3 to 6 days (mean 6.1 (1.37) days) after PCI | Cardiologist | Infusion into IRCA (AG) or IRCV (VG) | BMMNC | BM aspiration (**) | Saline solution and 5% human serum albumin | 100 million cells | No additional therapy (Control) |
| 4 to 11 days (median 9 days) after PCI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | n/r | 2,640 million cells | No additional therapy (Control) | |
| Piepoli 2010 | 4 to 7 days after AMI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Phosphate buffered saline ‐ EDTA and 5% human serum albumin | 249 million cells | No additional therapy (Control) |
| 3 to 11 days (mean 7 (2) days after AMI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Heparinised saline | 144 (49) million cells | No additional therapy (Control) | |
| Quyyumi 2011 | LD: median 191.4 (IQR 167 to 201) hours, MD: 210.0 (IQR 194 to 210) hours, HD: 207.3 (IQR 191 to 215) hours after AMI | Cardiologist | Infusion into IRCA | CD34‐positive cells | BM aspiration (**), immunomagnetic selection to isolate CD34‐positive cells | Heparinised phosphate buffered saline, 40% autologous serum and 1% human serum albumin | LD: 4.8 (0.4) million cells MD: 9.9 (0.7) million cells HD: 14.3 (1.6) million cells | No additional therapy (Control) |
| Roncalli 2010 | At 7 to 10 days (mean 9 (SD 1.7)) days | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | 4% human serum albumin solution | 98.3 (8.7) million cells | No additional therapy (Control) |
| Within 2 hours of successful PTCA | Cardiologist | Infusion into IRCA | BMMNC | n/r | Diluted autologous serum | n/r | Placebo (diluted autologous serum) | |
| Schachinger 2006 | Within 5 days (mean 4.3 (1.3) days) of PCI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | X‐VIVO medium and 20% autologous serum | 236 (174) million cells | Placebo (X‐VIVO medium and 20% autologous serum) |
| 5 to 12 days (mean 7 (2) days) after AMI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Heparinised saline | 900 (300) million | Placebo (heparinised saline) | |
| Sürder 2013 | 5 to 7 days (E) or 3 to 4 weeks (L) after PCI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Serum‐free medium and 20% of autologous serum | E: 159.7 (125.8) million cells L: 139.5 (120.5) million cells | No additional therapy (Control) |
| Tendera 2009 | Median 7 (IQR 3 to 12) days after PCI | Cardiologist | Infusion into IRCA | Selected cells (S): CD34/CXCR4‐ positive cells Unselected cells (U): BMMNC | BM aspiration (**). Selected cells: immunomagnetic selection to isolate CD34/CXCR4‐positive cells | Phosphate‐buffered saline | S: 1.9 million cells U: 178 million cells | No additional therapy (Control) |
| 3 to 10 days (median 4.5 (IQR 4 to 7) days) after PCI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | 0.9% saline solution and 5% human serum albumin | 100 million cells | Placebo (0.9% saline solution and 5% human serum albumin) | |
| Traverse 2011 | 2 to 3 weeks (median 17.5 (IQR 15.5 to 20.0) days) after AMI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | 0.9% saline solution and 5% human serum albumin | 147 (17) million cells | Placebo (0.9% saline solution and 5% human serum albumin) |
| Traverse 2012 | 3 days or 7 days after AMI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | 0.9% saline solution and 5% human serum albumin | 150 million cells | Placebo (0.9% saline solution and 5% human serum albumin) |
| 7 days after AMI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | n/r | n/r | No additional therapy (control) | |
| 15 (1) days after PCI | Cardiologist | Infusion into IRCA | BM‐MSC | BM aspiration (**) and culture of MSC | Heparinised saline | 100 million cells | Placebo (heparinised saline) | |
| Wohrle 2010 | 5 to 7 days (median 6.1 (IQR 5.5 to 7.3) days) after AMI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | 0.9% saline solution, 2% human serum albumin and 0.1% autologous erythrocytes | 381 (130) million cells | Placebo (0.9% saline solution, 2% human serum albumin and 0.1% autologous erythrocytes) |
| Wollert 2004 | 4.7 (1.3) days after PCI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Heparinised saline | 2460 (940) million cells | No additional therapy (Control) |
| Within 4 weeks of AMI | Cardiologist | Infusion into IRCA | BM‐MSC | BM aspiration (**) and culture of MSC | n/r | 460 (160) million cells | Placebo (heparinised saline) | |
| Within 7 days of AMI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Lymphocyte isolation medium | 210 (370) million cells | No additional therapy (control) | |
| SD: 3 to 7 days after PCI DD 3 to 7 days after PCI; second dose at 3 months | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Heparinised plasma | SD: 410 million cells DD: 190 (SE 120) million cells | Placebo (heparinised plasma) | |
| At day 14 | Cardiologist | Infusion into IRCA | BM‐MSC | BM aspiration (**), second centrifugation and culture of MSC | n/r | 75 million cells | No additional therapy (control) | |
| 14 to 19 days after AMI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Autologous serum | 50 million cells | No additional therapy (control) | |
| AMI ‐ acute myocardial infarction, PCI ‐ percutaneous coronary intervention, BM ‐ bone marrow, PTCA ‐ percutaneous transluminal coronary angioplasty, IRCA ‐ infarct‐related coronary artery, IRCV ‐ infarct‐related coronary vein, BMMNC ‐ bone marrow mononuclear cells, BM‐MSC ‐ mesenchymal stem cells; LD ‐ low dose, MD ‐ moderate dose, HD ‐ high dose, AG ‐ arterial group, VG ‐ venous group, E ‐ early cells, L ‐ late cells, S ‐ selected cells, U ‐ unselected cells, SD ‐ single dose, DD ‐ double dose ** BM aspiration‐ bone marrow aspiration and isolation of bone marrow mononuclear cells by gradient centrifugation | ||||||||
| Study ID | Primary Outcomes | Secondary Outcomes | ||||||||||||||||||||||
| All‐cause mortality | Cardiovascular mortality | Composite MACE (a) | Reinfarction | Hospital readmission for HF | Target vessel revascularisation | Arrhythmias | Restenosis | NYHA class | Quality of life (QoL) | Exercise tolerance | LVEF (b) | |||||||||||||
| ST | LT | ST | LT | ST | LT | ST | LT | ST | LT | ST | LT | ST | LT | ST | LT | ST | LT | ST | LT | ST | LT | ST | LT | |
| PR* | PR* | PR* | PR* | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | FR | |
| PR* | FR | NR | NR | NR | NR | PR* | PR* | NR | NR | PR* | FR | NR | NR | PR* | FR | NR | NR | NR | NR | NR | NR | FR | FR | |
| PR* | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | PR* | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | NR | |
| PR* | PR* | NR | PR* | NR | NR | NR | NR | FR | PR | NR | NR | NR | FR | NR | NR | NR | NR | NR | NR | NR | PR | FR | FR | |
| FR | FR | FR | FR | NR | FR | FR | FR | NR | FR | NR | NR | PR* | PR* | NR | NR | NR | NR | NR | NR | NR | NR | FR | FR | |
| PR* | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | PR* | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | NR | |
| NR | FR | NR | NR | NR | NR | FR | NR | NR | NR | FR | NR | NR | NR | FR | NR | NR | NR | NR | NR | FR | FR | FR | FR | |
| PR* | FR | NR | NR | FR | FR | FR | FR | FR | FR | FR | FR | FR | FR | NR | NR | NR | FR | NR | NR | NR | NR | FR | FR | |
| PR* | NR | NR | NR | NR | NR | PR* | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | NR | |
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | NR | |
| FR | NR | FR | NR | NR | NR | FR | NR | FR | NR | NR | NR | PR* | NR | PR | NR | NR | NR | NR | NR | FR | NR | FR | NR | |
| FR | NR | PR* | NR | NR | NR | NR | NR | NR | NR | PR* | NR | FR | NR | FR | NR | NR | NR | NR | NR | NR | NR | FR | FR | |
| PR* | NR | PR* | NR | NR | NR | PR* | NR | NR | NR | NR | NR | PR* | NR | PR* | NR | FR | NR | NR | NR | NR | NR | FR | NR | |
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | FR | FR | FR | NR | NR | FR | FR | |
| PR* | FR | PR* | FR | NR | NR | FR | FR | NR | NR | NR | NR | NR | NR | NR | NR | NR | PR | FR | NR | FR | NR | FR | NR | |
| PR* | NR | PR* | NR | NR | NR | FR | NR | NR | NR | PR* | NR | PR* | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | NR | |
| NR | FR | NR | NR | NR | NR | FR | FR | FR | FR | NR | FR | NR | FR | FR | NR | FR | NR | FR | NR | FR | NR | FR | FR | |
| PR* | PR* | PR* | PR* | NR | NR | FR | FR | FR | FR | NR | NR | PR* | NR | FR | PR | NR | NR | NR | NR | NR | NR | FR | FR | |
| FR | NR | PR* | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | PR | NR | NR | NR | NR | NR | NR | NR | FR | NR | |
| FR | FR | FR | FR | NR | FR | FR | FR | FR | FR | NR | NR | NR | PR* | NR | FR | NR | FR | NR | PR | NR | NR | FR | FR | |
| FR | FR | FR | FR | NR | NR | NR | NR | NR | NR | NR | NR | PR | NR | NR | FR | NR | NR | NR | NR | FR | PR | FR | FR | |
| FR | FR | FR | FR | NR | PR | FR | FR | NR | FR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | FR | |
| FR | FR | FR | FR | NR | NR | NR | NR | NR | FR | NR | FR | NR | PR* | NR | FR | NR | NR | NR | NR | NR | NR | FR | NR | |
| FR | PR | NR | NR | NR | NR | NR | NR | FR | NR | NR | NR | FR | NR | FR | NR | NR | NR | PR | PR | NR | NR | FR | PR | |
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | NR | |
| FR | FR | NR | FR | FR | FR | FR | FR | FR | FR | FR | FR | FR | FR | NR | NR | NR | NR | NR | NR | NR | NR | FR | FR | |
| PR* | NR | PR* | NR | NR | NR | PR* | NR | NR | NR | PR* | NR | PR* | NR | PR* | NR | NR | NR | NR | NR | NR | NR | FR | NR | |
| FR | PR | NR | NR | PR | PR | FR | NR | FR | NR | NR | NR | NR | NR | NR | NR | FR | NR | NR | NR | NR | NR | FR | FR | |
| FR | NR | NR | NR | NR | NR | FR | NR | NR | NR | FR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | NR | |
| PR* | NR | PR* | NR | NR | NR | NR | FR | NR | NR | NR | FR | NR | NR | NR | FR | NR | NR | NR | NR | NR | NR | FR | NR | |
| FR | NR | NR | NR | NR | NR | FR | NR | FR | NR | FR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | NR | |
| FR | FR | NR | NR | PR | PR | FR | FR | FR | FR | FR | FR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | FR | |
| PR* | NR | PR* | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | FR | NR | NR | NR | NR | FR | FR | |
| FR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | NR | |
| FR | NR | NR | NR | FR | FR | PR* | NR | FR | NR | PR* | NR | NR | NR | FR | NR | NR | NR | NR | NR | NR | NR | FR | FR | |
| PR* | FR | NR | FR | NR | FR | FR | FR | FR | FR | PR* | FR | NR | NR | FR | NR | NR | NR | NR | NR | NR | NR | FR | FR | |
| NR | NR | NR | NR | PR | NR | NR | NR | NR | NR | NR | NR | FR | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | NR | |
| NR | PR* | NR | PR* | NR | NR | NR | FR | NR | NR | NR | NR | NR | NR | FR | FR | NR | NR | NR | NR | NR | NR | FR | NR | |
| PR* | PR* | PR* | PR* | NR | NR | FR | FR | NR | NR | NR | NR | PR | PR | NR | NR | NR | NR | NR | NR | NR | NR | FR | FR | |
| PR* | NR | PR* | NR | NR | NR | NR | NR | NR | NR | NR | NR | PR* | NR | NR | NR | PR | NR | PR | NR | NR | NR | FR | NR | |
| FR | FR | FR | FR | NR | NR | NR | FR | NR | NR | NR | NR | NR | FR | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | |
| Total (%) analysed (c) | 1365 (50.0) | 996 (36.5) | 290 (10.6) | 527 (19.3) | 379 (13.9) | 497 (18.2) | 1521 (55.7) | 1116 (40.8) | 1194 (43.7) | 825 (30.2) | 789 (28.9) | 758 (27.7) | 525 (19.2) | 457 (16.7) | 641 (23.5) | 395 (14.4) | 398 (14.6) | 237 (8.7) | 154 (5.6) | 26 (1.0) | 267 (9.8) | 45 (1.6) | 1135 (41.5)(d) | 727 (26.6)(d) |
| ST ‐ short‐term follow‐up (< 12 months) LT ‐ long‐term follow‐up (≥ 12 months) FR ‐ full reporting, outcome included in analysis PR ‐ partial reporting, insufficient information on outcome reported for inclusion in analysis * no incidence of outcome observed NR ‐ outcome not reported HF ‐ heart failure; NYHA ‐ New York Heart Association; LVEF ‐ left ventricular ejection fraction (a)Composite measure of mortality, reinfarction or rehospitalisation for heart failure. (b)LVEF measured by any method. (c)Total number of participants included in meta‐analysis of outcome (% of total number of participants from all included studies). (d)Total number analysed given for LVEF measured by magnetic resonance imaging. | ||||||||||||||||||||||||
| Study ID | Number of analysed participants | All‐cause mortality events | Cardiovascular mortality events | Reinfarction | Target vessel revascularisation | Composite MACE (death, reinfarction, rehospitalisation for HF) | |||||||||||
| Cells | No cells | Cells | No cells | Length of follow‐up | Cells | No cells | Length of follow‐up | Cells | No cells | Length of follow‐up | Cells | No cells | Length of follow‐up | Cells | No cells | Length of follow‐up | |
| 11 | 11 | 0 | 0 | 12 months | 0 | 0 | 12 months | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ | |
| 41 | 45 | 0 | 1 | 48 months | NR | NR | ‐ | 0 | 0 | 48 months | 0 | 1 | 48 months | NR | NR | ‐ | |
| 34 | 35 | 0 | 0 | 6 months | 0 | 0 | 6 months | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ | |
| 5 | 4 | 0 | 0 | 12 months | 0 | 0 | 12 months | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ | |
| 21 | 21 | 1 | 0 | 24 months | 1 | 0 | 24 months | 1 | 0 | 24 months | NR | NR | ‐ | 2 | 1 | 24 months | |
| 10 | 10 | 0 | 0 | 6 months | 0 | 0 | 6 months | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ | |
| 27 | 12 | 1 | 0 | 12 months | NR | NR | ‐ | 1 (a) | 1 (a) | 6 months | 3 (a) | 4 (a) | 6 months | NR | NR | ‐ | |
| 65 | 60 | 1 | 2 | 60 months | NR | NR | ‐ | 1 | 1 | 60 months | 20 | 14 | 60 months | 2 | 5 | 60 months | |
| 20 | 20 | 0 | 0 | 6 months | 0 | 0 | 6 months | 0 | 0 | 6 months | NR | NR | ‐ | NR | NR | ‐ | |
| 20 | 20 | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ | |
| 40 | 40 | 0 | 1 | 6 months | 0 | 1 | 6 months | 0 | 2 | 6 months | NR | NR | ‐ | NR | NR | ‐ | |
| 33 | 34 | 1 | 0 | 4 months | 0 | 0 | 4 months | NR | NR | 4 months | NR | NR | ‐ | NR | NR | ‐ | |
| 16 | 16 | 0 | 0 | 6 months | 0 | 0 | 6 months | 0 | 0 | 6 months | NR | NR | ‐ | NR | NR | ‐ | |
| 14 | 12 | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ | |
| 26 | 32 | 10 | 4 | 8.2 years | 8 | 2 | 8.2 years | 2 | 2 | 8.2 years | NR | NR | ‐ | NR | NR | ‐ | |
| 30 | 28 | 0 | 0 | 6 months | 0 | 0 | 6 months | 2 | 0 | 6 months | 0 | 0 | 6 months | NR | NR | ‐ | |
| 49 | 50 | 1 | 1 | 36 months | NR | NR | ‐ | 1 | 2 | 36 months | 12 | 9 | 36 months | NR | NR | ‐ | |
| 44 | 20 | 0 | 0 | 12 months | 0 | 0 | 12 months | 2 | 0 | 12 months | NR | NR | ‐ | NR | NR | ‐ | |
| 24 | 6 | 1 | 0 | 6 months | 0 | 0 | 6 months | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ | |
| 17 | 10 | 3 | 0 | 24 months | 2 | 0 | 24 months | 1 | 1 | 24 months | NR | NR | ‐ | 6 | 5 | 24 months | |
| 19 | 19 | 2 | 4 | 12 months | 2 | 3 | 12 months | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ | |
| 40 | 20 | 2 | 2 | 24 months | 2 | 2 | 24 months | 1 | 1 | 24 months | NR | NR | ‐ | NR (c) | NR (c) | ‐ | |
| 16 | 15 | 1 | 0 | 12 months | 1 | 0 | 12 months | NR | NR | ‐ | 2 | 1 | 12 months | NR | NR | ‐ | |
| 48 | 44 | 1 | 0 | 3 months | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ | |
| 9 | 11 | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ | |
| 100 (b) | 100 (b) | 7 | 15 | 60 months | 5 | 9 | 60 months | 5 (b) | 7 (b) | 24 months | 18 (b) | 28 (b) | 60 months | 4 | 15 | 24 months | |
| 10 | 10 | 0 | 0 | 3 months | 0 | 0 | 3 months | 0 | 0 | 3 months | 0 | 0 | 3 months | NR | NR | ‐ | |
| 115 | 60 | 2 | 0 | 4 months | 0 | 0 | 4 months | 1 | 1 | 4 months | NR | NR | ‐ | NR (d) | NR (d) | ‐ | |
| 160 | 40 | 2 | 1 | 6 months | NR | NR | 3 | 2 | 6 months | 25 | 7 | 6 months | NR | NR | ‐ | ||
| 30 | 10 | 0 | 0 | 15 months | 0 | 0 | 15 months | 0 | 1 | 15 months | 0 | 1 | 15 months | NR | NR | ‐ | |
| 58 | 29 | 0 | 1 | 6 months | NR | NR | ‐ | 1 | 0 | 6 months | 1 | 2 | 6 months | NR | NR | ‐ | |
| 79 | 41 | 1 | 0 | 12 months | NR | NR | ‐ | 2 | 3 | 12 months | 4 | 4 | 12 months | NR (e) | NR (e) | ‐ | |
| 42 | 20 | 0 | 0 | 6 months | 0 | 0 | 6 months | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ | |
| 28 | 30 | 1 | 2 | 6 months | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ | |
| 29 | 13 | 1 | 1 | 6 months | NR | NR | ‐ | 0 | 0 | 6 months | 0 | 0 | 6 months | 5 | 1 | 36 months | |
| 30 | 30 | 2 | 2 | 61 months | NR | NR | ‐ | 1 | 1 | 61 months | 6 | 4 | 61 months | 5 | 6 | 61 months | |
| 17 | 21 | NR | NR | 3 months | NR | NR | 3 months | NR | NR | 3 months | NR | NR | 3 months | NR (f) | NR (f) | 3 months | |
| 90 | 84 | 0 | 0 | 30 months | 0 | 0 | 30 months | 2 | 2 | 30 months | NR | NR | ‐ | NR | NR | ‐ | |
| 27 | 12 | 0 | 0 | 12 months | 0 | 0 | 12 months | 0 | 1 | 12 months | NR | NR | ‐ | NR | NR | ‐ | |
| 7 | 16 | 0 | 0 | 8 weeks | 0 | 0 | 8 weeks | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ | |
| 8 | 3 | 2 | 1 | 36 months * | 2 | 1 | 36 months * | 1 | 0 | 36 months | NR | NR | ‐ | NR | NR | ‐ | |
| (a)Grajek 2010: 31 BMMNC and 14 controls available for analysis at 6 months. (b)Schachinger 2006: 100 BMMNC and 101 controls analysed at 24 months; 3 patients (2 BMMNC and 1 control) only had mortality data at 60 months. (c)Plewka 2009: Composite death, MI, hospitalisation for HF, TVR: 9 BMMNC and 11 controls at 24 months. (d)Sürder 2013: Composite death, MI, revascularisation, hospitalisation for HF: 9 BMMNC and 8 controls at 12 months. (e)Traverse 2012: Composite death, MI, hospitalisation for HF, revascularisation, ICD, stroke: 18 BMMNC and 9 controls at 12 months. (f)Xiao 2012: Composite MACE (undefined): 3 BMMNC and 2 controls at 3 months. | |||||||||||||||||
| Study ID | Periprocedural adverse events |
| Not reported | |
| 1 x transient acute heart failure 7 days after cell transplantation | |
| Not reported | |
| No adverse events were reported until the end of hospitalisation | |
| 1 x death 3 days after cell transplantation due to suspected acute in‐stent thrombosis; 1 x serious complication of acute coronary occlusion during cell injection with subsequent recurrent MI | |
| No bleeding complications at BM puncture site and no angina aggravation, malignant diseases or substantial arrhythmias after PCI and BM transfer during hospitalisation in either treatment group | |
| Not reported | |
| No complications of cell harvesting. A CK or CK‐MB elevation between 1 and 2 times the ULN was detected in 4 patients and between 2 and 3 times the ULN in one patient. 1 x occluded infarct‐related artery (patient did not receive cell therapy as randomised). During cell catheterisation: 1 x coronary spasm, 1 x transient brachycardia and 1 x thrombus in the infarct related artery | |
| Not reported | |
| Not reported | |
| 3 x mild self terminating vasovagal reactions during BM aspiration; no other procedural complications relating to aspiration. Subacute stent thrombosis occurred in 4 patients (1 x cell therapy and 3 x placebo); 1 x cell therapy patient had 'no reflow' phenomenon after stenting of the infarcted artery | |
| 11 x treatment‐related tachycardia (supraventricular arrhythmia: 5 in the cell therapy group and 6 in the control group); 3 patients in the control group experienced non‐sustained ventricular tachycardia | |
| Not reported | |
| Not reported | |
| No complications of BM aspiration or cell infusion | |
| No serious inflammatory reactions or bleeding complications from BM aspiration. No (or mild) angina during balloon inflation. No serious procedural complications related to intracoronary administration of MSCs including ventricular arrhythmia, thrombus formation or dissection. Periprocedural MI occurred in 2 patients | |
| 2 x stent thrombosis in the acute phase in the cell therapy group (no cells administered as randomised); 1 x sustained ventricular tachycardia before cell administration; 1 x ventricular fibrillation at day 6, 24 hours after injection.1 x pulseless ventricular tachycardia in control patient ‐ converted to sinus rhythm by means of a precordial thump on day 2 | |
| 2 patients had fever and 1 patient had brachycardia, all within 20 hours prior to cells (these patients did not receive cell therapy as randomised). 3 x cell therapy‐related complications: 1 x intimal dissection during repeat balloon inflations at time of cell implantation, 1 x short‐lasting fever on day of scheduled transplantation, 1 x small thrombus in infarct‐related artery diagnosed immediately after cell transplantation. 2 x control patients had repeat MI 2 days after the hospital discharge due to in‐stent thrombosis | |
| Ck‐MB elevation (3 x normal value) in 3 patients in the arterial group and 1 patient in venous group. 1 x tortuous anterior interventricular vein (patient did not receive cell therapy as randomised). No new pericardial effusions | |
| 2 x serious complications (1 x stent thrombosis with reinfarction immediately after BM harvest, patient died 2 weeks later due to sepsis and acute respiratory distress syndrome; 1 x ventricular septal rupture before cell injection, patient died 3 months later from severe heart failure). | |
| All procedures well tolerated. No inflammatory reaction or abscess detected at the site of puncture after BM harvest. The invasive coronary catheterisation was associated with some mild angina during balloon inflations for cell infusions. No procedural complications during cardiac catheterisation related to cell injections (no ventricular arrhythmia, new thrombus formation or embolism after cell infusion or dissections due to balloon inflations) | |
| Not reported | |
| 1 high‐dose treatment group patient died soon after cell infusion from ventricular fibrillation attributed to recurrent MI from stent thrombosis preceding cell infusion. 1 x high‐dose treatment group patient with acute stent thrombosis before cell infusion (patient withdrawn from study). Cell therapy group: 1 x arrhythmia, 1 x chest pain, 3 x musculoskeletal pain, 2 x upper respiratory tract infection, 2 x rash, 3 x dyspnoea, 1 x fever. Control group: 1 x arrhythmia, 3 x musculoskeletal pain, 1 x upper respiratory tract infection, 1 x dyspnoea | |
| Cell therapy group: 1 x transient ischaemic attack and 1 x thrombopenia induced by GP2b3a inhibitor (both excluded before BM aspiration). Control group: 1 x steroids given for angioneurotic oedema; 1 x post‐MI ventricular septal defect (both withdrawn before day 7) | |
| Not reported | |
| No bleeding complications or haematoma formation at puncture site of BM aspiration. 1 x patient was excluded owing to fever and an increase in the level of C‐reactive protein. 1 x patient in placebo group had angiographic evidence of a thrombus in a non‐infarct‐related artery (placebo medium not infused). 2 x deaths, cause not reported (1 x cell therapy group and 1 x placebo) and 2 x reinfarction (cell therapy group) prior to discharge | |
| Not reported | |
| 1 death in cell therapy group prior to transplantation, cause of death not reported | |
| 1 patient developed arteriovenous fistula of the femoral artery after the procedure and required surgical treatment. No complications arising from BM cell transfer | |
| BM aspiration carried out without complications. No patient experienced a rise in troponin or procedure‐related complication following infusion | |
| No complications associated with BM aspiration. 2 x patients underwent additional stenting at time of cell infusion (1 x distal stent edge dissection related to primary PCI procedure; 1 x possible dissection related to stop‐flow procedure). 1 x postpartum spontaneous coronary dissection with diffuse thrombus throughout stented region of left anterior descending artery; 1 x presence of severe left main coronary stenosis identified before transfusion (this patient did not receive cell therapy as randomised). No patients experienced postprocedural increase in cardiac enzymes | |
| No complications associated with BM harvesting or intracoronary infusion. 1 x death in the BM cell therapy group due to subarachnoid haemorrhage prior to cell delivery | |
| No procedural or cell‐induced complications and no side effects in any patient | |
| Not reported | |
| Not reported | |
| No bleeding complications at BM harvest site. No increases in troponin T serum levels in any patients 24 hours after BM transfer | |
| Not reported | |
| 1 x temporary hypotension, 2 x brachycardia, 7 x new hyperuricaemia | |
| 1 x brachycardia with subsequent pacemaker implantation, 1 x fever (these patients did not receive cells as randomised) | |
| Not reported | |
| Not reported | |
| MI, acute myocardial infarction; PCI, percutaneous coronary intervention; BM, bone marrow; MSC, mesenchymal stem cells; ULN, upper limit of normal | |
| Study ID | No. analysed participants | Quality of life (QoL) assessment | Reported data (EP/MC/SR) | Performance assessment | Summary measures of performance | Reported data (EP/MC/SR) | Mean follow‐up | |
| Cells | No cells | |||||||
| 5 | 4 | n/r | n/r | Exercise stress test | Peak HR, peak MET, peak double product (SBPxHR), peak predicted HR | EP (median) | 12 months | |
| 31 | 14 | n/r | n/r | Cardiopulmonary exercise treadmill test (modified Bruce protocol) | METs, maximum VO2 , VE/VCO2 slope, RER, peak SBP, peak HR, VO2 anaerobic threshold, HR recovery | EP | 12 months | |
| 65 | 60 | n/r | n/r | NYHA class | EP | 60 months | ||
| 27 | 27 | n/r | n/r | Symptom‐limited maximal exercise test | METs, peak HR, T‐wave alternans | EP, MC | 6 months | |
| 16 | 16 | n/r | n/r | NYHA class | EP | 6 months | ||
| 14 | 12 | MLHFQ | EP | NYHA class | EP | 12 months | ||
| 16 (a) | 28 (a) | MLHFQ | EP | Six minute walk test; functional class (undefined) | Distance (metres) | EP | 6 months | |
| 50 (b) | 50 (b) | SF‐36 | EP, MC | Electrically braked bicycle ergometer; NYHA class | Time (min), maximum VO2 , VE/VCO2 slope etc., peak HR | EP, MC | 6 months | |
| 14 | 10 | SF‐36 | SR | NYHA class | EP | 24 months | ||
| 17 | 15 | n/r | n/r | Cardiopulmonary exercise treadmill test (modified Bruce protocol) | Exercise duration (min), maximum VO2 , VE/VCO2 slope | MC | 12 months | |
| 52 | 49 | MLHFQ | SR | n/r | 12 months | |||
| 117 | 61 | n/r | n/r | NYHA class | EP | 4 months | ||
| 42 | 20 | n/r | n/r | NYHA class | EP | 12 months | ||
| 7 | 16 | QoL (no details) | NYHA class | SR | 8 weeks | |||
| MLHFQ, Minnesota Living with Heart Failure Questionnaire; NYHA, New York Heart Association; SF‐36, Short‐Form 36 Quality of Life; MET, metabolic equivalent test (mL/kg/min); HR, heart rate (bpm); SBP, systolic blood pressure (mmHg); RER, respiratory exchange ratio; VE, minute ventilation; VO2, oxygen volume; VCO2, carbon dioxide volume; EP, endpoint; MC, mean change from baseline; SR, summary results; n/r, not reported. (a)Karpov 2005: QoL was measured in 37 participants (cells: 18 cells, no cells: 19) (b)Lunde 2006: QoL was measured in 46 BMMNC and 45 controls; exercise tolerance was measured in 49 BMMNC and 50 controls | ||||||||
| Study ID | No. randomised participants | No. analysed participants | Baseline LVEF | Mean follow‐up of LVEF | |||
| Cells | No cells | Cells | No cells | Cells | No cells | ||
| Measured by MRI | |||||||
| Hirsch 2011 (HEBE) | 69 | 65 | 59 | 52 | 43.7 (9.0)% | 42.4 (8.3)% | 24 months |
| 20 | 20 | 20 | 20 | 44.5 (7.1)% | 43.4 (6.7)% | 6 months | |
| 33 | 34 | 30 | 30 | 48.5 (7.2)% | 46.9 (8.2)% | 12 months | |
| Lunde 2006 (ASTAMI) | 50 | 51 | 44 | 44 | 54.8 (13.6)% | 53.6 (11.6)% | 36 months |
| Quyyumi 2011 (AMR‐1) | 16 | 15 | 11 | 10 | LD: 47.0 (13)% MD: 47.3 (11)% HD: 49.9 (7)% | 53.2(10)% | 6 months |
| Roncalli 2010 (BONAMI) | 52 | 49 | 47 | 43 | 37.0 (9.8)% | 38.7 (9.2)% | 3 months |
| Schachinger 2006 (REPAIR‐AMI) | 101 | 103 | 26 | 33 | 47.8 (6.2)% | 47.7 (6.2)% | 60 months (a) |
| Sürder 2013 (SWISS‐AMI) | 133 | 67 | 107 | 60 | E: 36.5 (9.9)% L: 36.3 (8.2)% | 40.0 (9.9)% | 4 months |
| Tendera 2009(REGENT) | 160 | 40 | 97 | 20 | S: 33.9 (8.6)% U: 35.6 (6.5)% | 38.9 (5.2)% | 6 months |
| 30 | 10 | 30 | 10 | 49 (9.5)% | 48.6 (8.5)% | 6 months | |
| Traverse 2011 (LATE‐TIME) | 59 | 29 | 55 | 26 | 48.7 (12)% | 45.3 (9.9)% | 6 months |
| Traverse 2012 (TIME) | 80 | 40 | 65 | 30 | 46.2 (9.6)% | 46.3 (8.5)% | 12 months |
| Wohrle 2010 (SCAMI) | 29 | 13 | 28 | 12 | 53.5 (9.3)% | 55.7 (9.4)% | 36 months |
| Wollert 2004 (BOOST) | 33 | 32 | 30 | 30 | 50 (10)% | 51.3 (9.3)% | 60 months |
| 30 | 15 | 27 | 11 | SD: 32.5 (3.6)% DD: 33.7 (4.7)% | 32.3 (2.0)% | 12 months | |
| 8 | 3 | 6 (b) | 1 (b) | 33.4 (3)% | 28 (4)% | 36 months (b) | |
| Measured by echocardiography | |||||||
| 11 | 11 | 11 | 11 | n/r | n/r | 12 months | |
| 41 | 45 | 41 | 45 | 41.3 (2.8)% | 40.7 (3.1)% | 48 months | |
| 5 | 5 | 5 | 4 | 44.6 (8.8)% | 43.2 (9.1)% | 12 months | |
| 21 | 22 | 19 | 20 | 50.8 (6.5)% | 51.4 (7.2)% | 24 months | |
| 10 | 10 | 10 | 10 | 53.8 (9.2)% | 58.2 (7.5)% | 6 months | |
| 31 | 14 | 27 | 12 | 50.3 (9.8)% | 50.8 (12)% | 12 months | |
| 20 | 20 | 20 | 20 | 48.5 (5.5)% | 48.2 (6.30% | 6 months | |
| Huikuri 2008 (FINCELL) | 40 | 40 | 39 | 38 | 56 (10)% | 57 (10)% | 6 months |
| 14 | 12 | 14 | 12 | 54.3 (5.5)% | 55.8 (5.9)% | 12 months | |
| 22 | 22 | 16 | 10 | 49.3 (11.1)% | 47.0 (7.5)% | 6 months | |
| Lee 2014 (SEED‐MSC) | 40 | 40 | 30 | 28 | 48.1 (8.0)% | 51.0 (9.2)% | 6 months |
| Lunde 2006 (ASTAMI) | 50 | 51 | 50 | 50 | 45.7 (9.4)% | 46.9 (8.6)% | 36 months |
| Nogueira 2009 (EMRTCC) | 24 | 6 | 22 | 6 | AG: 48.3 (10.4)% VG: 48.6 (7.1)% | 47.6 (14.3)% | 6 months |
| 17 | 10 | 14 | 10 | 39.2 (9.2)% | 39.4 (5.6)% | 24 months | |
| Piepoli 2010 (CARDIAC) | 19 | 19 | 17 | 15 | 38.4 (6.4)% | 38.9 (5.6)% | 24 months |
| 40 | 20 | 38 | 18 | 35 (6)% | 33 (7)% | 24 months | |
| Roncalli 2010 (BONAMI) | 52 | 49 | 47 | 43 | 38.1 (7.9)% | 39.8 (7.0)% | 12 months (c) |
| 9 | 11 | 9 | 11 | 53.4 (8.9)% | 53.5 (5.8)% | 6 months | |
| 17 | 21 | 17 | 21 | 35.6 (3.1)% | 35.7 (3.1)% | 3 months | |
| 7 | 16 | 7 | 16 | 37 (4.6)% | 38.6 (5.4)% | 8 weeks | |
| Measured by SPECT | |||||||
| 11 | 11 | 11 | 11 | n/r | n/r | 12 months | |
| 41 | 45 | 41 | 45 | 41.2 (3.1)% | 40.8 (3.3)% | 48 months | |
| Lee 2014 (SEED‐MSC) | 40 | 40 | 30 | 28 | 49.0 (11.7)% | 52.3 (9.3)% | 6 months |
| Lunde 2006 (ASTAMI) | 50 | 51 | 50 | 50 | 41.3 (10.4)% | 42.6 (11.7)% | 6 months |
| 44 | 22 | 40 | 20 | LD: 41 (2)% HD: 30 (2)% | 40 (2)% | 12 months | |
| Piepoli 2010 (CARDIAC) | 19 | 19 | 17 | 15 | 36.6 (8.2)% | 37.5 (8.9)% | 24 months |
| 40 | 20 | 26 | 10 | 41.2 (10.1)% | 40.0 (14.2)% | 6 months | |
| Measured by LV angiography | |||||||
| 34 | 35 | 34 | 35 | 49 (9)% | 48 (10)% | 6 months | |
| 20 | 20 | 20 | 20 | 56.7 (9.7)% | 57.3 (8.2)% | 6 months | |
| Huikuri 2008 (FINCELL) | 40 | 40 | 36 | 36 | 59 (11)% | 62 (12)% | 6 months |
| n/r | n/r | 16 | 16 | 33.37 (11.2)% | 29.0 (7.5)% | 6 months | |
| Schachinger 2006 (REPAIR‐AMI) | 101 | 103 | 95 | 92 | 48.3 (9.2)% | 46.9 (10.4)% | 4 months |
| 10 | 10 | 10 | 10 | 37 (5)% | 39 (6)% | 3 months | |
| 42 | 20 | 42 | 20 | 43 (10)% | 45 (10)% | 12 months | |
| 30 | 30 | 27 | 28 | 37.8 (6.3)% | 20.2 (2.5)% (d) | 6 months | |
| 92 | 92 | 90 | 84 | n/r | n/r | 6 months | |
| Measured by RNV | |||||||
| 31 | 14 | 27 | 12 | 45.4 (10.2)% | 42.7 (7.4)% | 12 months | |
| Nogueira 2009 (EMRTCC) | 24 | 6 | 22 | 6 | AG: 41.0 (10.3)% VG: 39.9 (7.4)% | 40.1 (12.4)% | 6 months |
| Roncalli 2010 (BONAMI) | 52 | 49 | 47 | 43 | 35.6 (7.0)% | 37.0 (6.7)% | 3 months |
| Measured by gated PET | |||||||
| 5 | 5 | 5 | 4 | 36.6 (5.4)% | 37.6 (7.0)% | 12 months | |
| n/r ‐ not reported LD ‐ low dose, MD ‐ moderate dose, HD ‐ high dose, AG ‐ arterial group, VG ‐ venous group, E ‐ early cells, L ‐ late cells, S ‐ selected cells, U ‐ unselected cells, SD ‐ single dose, DD ‐ double dose (a)Schachinger 2006: MRI was performed at five‐year follow‐up but summary results only were reported; 24‐month data are used in meta‐analysis. (b)Zhukova 2009: 24‐month data were used in the analysis as only one control was available at 36 months. (c)Roncalli 2010: echocardiography was performed at 12‐month follow‐up but summary results only were reported; three‐month data are used in meta‐analysis. (d)Wang 2014: the reported baseline LVEF value in the control group is assumed to be an error since the difference between values at baseline and endpoint (49.1%) is not significant. We have been unable to clarify the correct value with the study authors. | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 23 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Short‐term follow‐up (< 12 months) | 17 | 1365 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.43, 1.49] |
| 1.2 Long‐term follow‐up (≥ 12 months) | 14 | 996 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.58, 1.50] |
| 2 Cardiovascular mortality Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Short‐term follow‐up (< 12 months) | 7 | 290 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.28, 1.82] |
| 2.2 Long‐term follow‐up (≥ 12 months) | 9 | 527 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.54, 1.99] |
| 3 Composite measure of death, reinfarction, re‐hospitalisation for heart failure Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Short‐term follow‐up (< 12 months) | 3 | 379 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.12, 1.14] |
| 3.2 Long‐term follow‐up (≥ 12 months) | 6 | 497 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.36, 1.10] |
| 4 Incidence of reinfarction Show forest plot | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Short‐term follow‐up (< 12 months) | 17 | 1521 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.33, 1.30] |
| 4.2 Long‐term follow‐up (≥ 12 months) | 14 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.36, 1.12] |
| 5 Incidence of re‐hospitalisation for heart failure Show forest plot | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Short‐term follow‐up (< 12 months) | 13 | 1194 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.40, 1.62] |
| 5.2 Long‐term follow‐up (≥ 12 months) | 10 | 825 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.30, 1.00] |
| 6 Incidence of target vessel revascularisation Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Short‐term follow‐up (< 12 months) | 6 | 789 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.47, 1.06] |
| 6.2 Long‐term follow‐up (≥ 12 months) | 8 | 758 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.67, 1.37] |
| 7 Incidence of arrhythmias Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Short‐term follow‐up (< 12 months) | 5 | 525 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.51, 1.98] |
| 7.2 Long‐term follow‐up (≥ 12 months) | 5 | 457 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.58, 3.37] |
| 8 Incidence of restenosis Show forest plot | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Short‐term follow‐up (< 12 months) | 8 | 641 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.63, 1.43] |
| 8.2 Long‐term follow‐up (≥ 12 months) | 6 | 395 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.27, 1.25] |
| 9 Quality of life measures Show forest plot | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Short‐term follow‐up (< 12 months) | 3 | 154 | Std. Mean Difference (IV, Random, 95% CI) | 0.58 [‐0.67, 1.83] |
| 9.2 Long‐term follow‐up (≥ 12 months) | 1 | 26 | Std. Mean Difference (IV, Random, 95% CI) | 3.23 [2.01, 4.46] |
| 10 NYHA classification Show forest plot | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 Short‐term follow‐up (< 12 months) | 5 | 398 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.24, 0.09] |
| 10.2 Long‐term follow‐up (≥ 12 months) | 4 | 237 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.53, 0.07] |
| 11 Exercise tolerance Show forest plot | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 Short‐term follow‐up (< 12 months) | 5 | 267 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.06, 0.43] |
| 11.2 Long‐term follow‐up (≥ 12 months) | 1 | 45 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.68, 0.58] |
| 12 Maximum VO2 (mL/kg/min) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 Short‐term follow‐up (< 12 months) | 3 | 175 | Mean Difference (IV, Random, 95% CI) | 1.15 [‐0.77, 3.07] |
| 12.2 Long‐term follow‐up (≥ 12 months) | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐3.76, 4.56] |
| 13 VE/VCO2 slope Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1 Short‐term follow‐up (< 12 months) | 3 | 174 | Mean Difference (IV, Random, 95% CI) | 0.28 [‐1.02, 1.57] |
| 13.2 Long‐term follow‐up (≥ 12 months) | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐3.07, 3.07] |
| 14 Peak heart rate (bpm) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1 Short‐term follow‐up (< 12 months) | 3 | 198 | Mean Difference (IV, Random, 95% CI) | 0.55 [‐6.79, 7.89] |
| 14.2 Long‐term follow‐up (≥ 12 months) | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐9.10 [‐20.59, 2.39] |
| 15 LVEF measured by MRI (<12 months) Show forest plot | 15 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.1 Mean change from baseline | 13 | 1057 | Mean Difference (IV, Random, 95% CI) | 0.43 [‐1.16, 2.03] |
| 15.2 Mean value at endpoint | 15 | 1125 | Mean Difference (IV, Random, 95% CI) | 0.81 [‐0.78, 2.41] |
| 15.3 Combined | 15 | 1135 | Mean Difference (IV, Random, 95% CI) | 1.05 [‐0.56, 2.67] |
| 16 LVEF measured by MRI (≥ 12 months) Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.1 Mean change from baseline | 5 | 438 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐1.72, 1.78] |
| 16.2 Mean value at endpoint | 8 | 551 | Mean Difference (IV, Random, 95% CI) | 1.40 [‐1.54, 4.34] |
| 16.3 Combined | 9 | 718 | Mean Difference (IV, Random, 95% CI) | 1.27 [‐1.14, 3.68] |
| 17 LVEF measured by echocardiography (< 12 months) Show forest plot | 20 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 17.1 Mean change from baseline | 6 | 372 | Mean Difference (IV, Random, 95% CI) | 2.72 [1.50, 3.95] |
| 17.2 Mean value at endpoint | 20 | 862 | Mean Difference (IV, Random, 95% CI) | 2.15 [0.89, 3.42] |
| 17.3 Combined | 20 | 862 | Mean Difference (IV, Random, 95% CI) | 2.31 [1.30, 3.33] |
| 18 LVEF measured by echocardiography (≥12 months) Show forest plot | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 18.1 Mean change from baseline | 3 | 127 | Mean Difference (IV, Random, 95% CI) | 1.35 [‐2.25, 4.96] |
| 18.2 Mean value at endpoint | 9 | 377 | Mean Difference (IV, Random, 95% CI) | 2.87 [1.42, 4.31] |
| 18.3 Combined | 10 | 433 | Mean Difference (IV, Random, 95% CI) | 2.09 [0.74, 3.44] |
| 19 LVEF measured by SPECT (< 12 months) Show forest plot | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 19.1 Mean change from baseline | 5 | 286 | Mean Difference (IV, Random, 95% CI) | 2.72 [0.23, 5.21] |
| 19.2 Mean value at endpoint | 6 | 375 | Mean Difference (IV, Random, 95% CI) | 2.19 [0.58, 3.81] |
| 19.3 Combined | 7 | 394 | Mean Difference (IV, Random, 95% CI) | 2.52 [0.59, 4.44] |
| 20 LVEF measured by SPECT (≥ 12 months) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 20.1 Mean change from baseline | 2 | 92 | Mean Difference (IV, Random, 95% CI) | 5.63 [1.77, 9.49] |
| 20.2 Mean value at endpoint | 3 | 181 | Mean Difference (IV, Random, 95% CI) | 3.46 [0.82, 6.11] |
| 20.3 Combined | 4 | 200 | Mean Difference (IV, Random, 95% CI) | 4.42 [2.68, 6.16] |
| 21 LVEF measured by left ventricular angiography (< 12 months) Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 21.1 Mean change from baseline | 3 | 279 | Mean Difference (IV, Random, 95% CI) | 6.43 [0.60, 12.27] |
| 21.2 Mean value at endpoint | 9 | 711 | Mean Difference (IV, Random, 95% CI) | 4.94 [0.53, 9.35] |
| 21.3 Combined | 9 | 711 | Mean Difference (IV, Random, 95% CI) | 5.09 [0.95, 9.24] |
| 22 LVEF measured by left ventricular angiography (≥ 12 months) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 22.1 Mean value at endpoint | 1 | 62 | Mean Difference (IV, Random, 95% CI) | 8.0 [4.27, 11.73] |
| 23 LVEF measured by radionuclide ventriculography (RNV) (<12 months) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 23.1 Mean change from baseline | 2 | 118 | Mean Difference (IV, Random, 95% CI) | 0.91 [‐3.11, 4.94] |
| 23.2 Mean value at endpoint | 3 | 157 | Mean Difference (IV, Random, 95% CI) | 1.08 [‐4.88, 7.04] |
| 23.3 Combined | 3 | 157 | Mean Difference (IV, Random, 95% CI) | 1.79 [‐1.86, 5.43] |
| 24 LVEF measured by radionuclide ventriculography (≥ 12 months) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 24.1 Mean value at endpoint | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 6.30 [‐1.03, 13.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality (< 12 months) Show forest plot | 16 | 1335 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.42, 1.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality (< 12 months) Show forest plot | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Excluding studies with high risk of selection bias | 16 | 1307 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.43, 1.57] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality (< 12 months) Show forest plot | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Excluding studies with a high or unclear risk of attrition bias | 13 | 899 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.38, 1.61] |
| 2 All‐cause mortality (≥ 12 months) Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Excluding studies with a high or unclear risk of attrition bias | 11 | 847 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.38, 1.17] |
| 3 Cardiovascular mortality (< 12 months) Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Excluding studies with high or unclear risk of attrition bias | 5 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.22, 2.14] |
| 4 Cardiovascular mortality (≥ 12 months) Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Excluding studies with high or unclear risk of attrition bias | 6 | 378 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.34, 1.50] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality (< 12 months) Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Excluding studies with a high risk of performance bias | 8 | 669 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.23, 1.56] |
| 2 All‐cause mortality (≥ 12 months) Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Excluding studies with a high risk of performance bias | 3 | 406 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.22, 1.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality (< 12 months) Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Baseline LVEF < 45% | 4 | 478 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.19, 3.16] |
| 1.2 Baseline LVEF ≥ 45% | 6 | 551 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.32, 2.98] |
| 2 All‐cause mortality (≥ 12 months) Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Baseline LVEF < 45% | 2 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.13, 2.83] |
| 2.2 Baseline LVEF ≥ 45% | 5 | 510 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.31, 1.30] |
| 3 LVEF measured by MRI (< 12 months) Show forest plot | 15 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Baseline LVEF < 45% | 6 | 579 | Mean Difference (IV, Random, 95% CI) | 2.28 [0.43, 4.13] |
| 3.2 Baseline LVEF ≥ 45% | 9 | 556 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐2.42, 2.24] |
| 4 LVEF measured by MRI (≥ 12 months) Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Baseline LVEF < 45% | 4 | 326 | Mean Difference (IV, Random, 95% CI) | 3.93 [‐0.15, 8.02] |
| 4.2 Baseline LVEF ≥ 45% | 5 | 342 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐2.34, 2.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality (< 12 months) Show forest plot | 17 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Mononuclear cells | 14 | 1153 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.38, 1.46] |
| 1.2 Mesenchymal stem cells | 2 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.15, 6.60] |
| 1.3 Haematopoietic progenitor cells | 2 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.13, 8.36] |
| 2 All‐cause mortality (≥ 12 months) Show forest plot | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Mononuclear cells | 12 | 923 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.54, 1.43] |
| 2.2 Mesenchymal stem cells | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 69.70] |
| 2.3 Haematopoietic progenitor cells | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 2.82 [0.12, 64.39] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality (< 12 months) Show forest plot | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 ≤ 108 cells | 5 | 297 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.27, 3.96] |
| 1.2 > 108 and ≤ 109 cells | 12 | 1081 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.33, 1.34] |
| 2 All‐cause mortality (≥ 12 months) Show forest plot | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 ≤ 108 cells | 5 | 241 | Risk Ratio (M‐H, Random, 95% CI) | 2.20 [0.97, 4.95] |
| 2.2 > 108 and ≤ 109 cells | 7 | 668 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.28, 0.97] |
| 2.3 > 109 cells | 2 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.32, 7.55] |
| 3 LVEF measured by MRI (< 12 months) Show forest plot | 14 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 ≤ 108 cells | 4 | 270 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐3.51, 3.52] |
| 3.2 > 108 and ≤ 109 cells | 11 | 825 | Mean Difference (IV, Random, 95% CI) | 1.08 [‐0.53, 2.69] |
| 4 LVEF measured by MRI (≥ 12 months) Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 ≤ 108 cells | 2 | 98 | Mean Difference (IV, Random, 95% CI) | 3.60 [‐4.24, 11.44] |
| 4.2 > 108 and ≤ 109 cells | 7 | 570 | Mean Difference (IV, Random, 95% CI) | 1.48 [‐1.44, 4.40] |
| 5 LVEF measured by left ventricular angiography (< 12 months) Show forest plot | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 > 108 and ≤ 109 cells | 6 | 548 | Mean Difference (IV, Random, 95% CI) | 2.26 [‐0.71, 5.23] |
| 5.2 > 109 cells | 2 | 101 | Mean Difference (IV, Random, 95% CI) | 11.64 [7.52, 15.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality (< 12 months) Show forest plot | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 ≤ 10 days since AMI | 10 | 839 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.45, 2.30] |
| 1.2 > 10 days since AMI | 3 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.06, 1.36] |
| 2 All‐cause mortality (≥ 12 months) Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 ≤ 10 days since AMI | 9 | 809 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.33, 1.11] |
| 2.2 > 10 days since AMI | 1 | 11 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.10, 5.54] |
| 3 LVEF measured by MRI (< 12 months) Show forest plot | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 ≤ 10 days since AMI | 12 | 867 | Mean Difference (IV, Random, 95% CI) | 1.15 [‐0.66, 2.97] |
| 3.2 > 10 days since AMI | 2 | 190 | Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐4.90, 3.48] |
| 4 LVEF measured by MRI (≥ 12 months) Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 ≤ 10 days since AMI | 9 | 669 | Mean Difference (IV, Random, 95% CI) | 1.26 [‐1.20, 3.71] |
| 4.2 > 10 days since AMI | 1 | 109 | Mean Difference (IV, Random, 95% CI) | 1.17 [‐2.59, 4.93] |
| 5 LVEF measured by left ventricular angiography (< 12 months) Show forest plot | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 ≤ 10 days since AMI | 5 | 535 | Mean Difference (IV, Random, 95% CI) | 2.20 [‐1.51, 5.91] |
| 5.2 > 10 days since AMI | 3 | 156 | Mean Difference (IV, Random, 95% CI) | 7.42 [‐1.83, 16.66] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality (< 12 months) Show forest plot | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Heparin | 6 | 339 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.31, 2.66] |
| 1.2 No heparin | 10 | 999 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.30, 1.45] |
| 2 All‐cause mortality (≥ 12 months) Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Heparin | 7 | 503 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.33, 2.10] |
| 2.2 No heparin | 5 | 408 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.28, 1.08] |
| 3 LVEF measured by MRI (< 12 months) Show forest plot | 15 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Heparin | 7 | 434 | Mean Difference (IV, Random, 95% CI) | 1.99 [‐0.62, 4.59] |
| 3.2 No heparin | 8 | 701 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐1.67, 2.17] |
| 4 LVEF measured by MRI (≥ 12 months) Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Heparin | 5 | 357 | Mean Difference (IV, Random, 95% CI) | 1.76 [‐1.93, 5.45] |
| 4.2 No heparin | 4 | 361 | Mean Difference (IV, Random, 95% CI) | 0.53 [‐2.14, 3.20] |
| 5 LVEF measured by left ventricular angiography (< 12 months) Show forest plot | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Heparin | 5 | 256 | Mean Difference (IV, Random, 95% CI) | 6.82 [0.25, 13.39] |
| 5.2 No heparin | 3 | 393 | Mean Difference (IV, Random, 95% CI) | 1.91 [‐3.46, 7.27] |