Anticoagulation for people with cancer and central venous catheters
Information
- DOI:
- https://doi.org/10.1002/14651858.CD006468.pub6Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 01 June 2018see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Gynaecological, Neuro-oncology and Orphan Cancer Group
- Copyright:
-
- Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
LAK: searching for trials, full‐text retrieval, screening, data extraction, data analysis, data interpretation, manuscript drafting, review co‐ordination.
IGT: screening, full‐text retrieval, data extraction, manuscript drafting.
MBH: full‐text retrieval, screening, data extraction.
CM: screening, full‐text retrieval, data extraction.
MB: screening, full‐text retrieval, data extraction.
VY: screening, full‐text retrieval, data extraction.
IT: screening, full‐text retrieval, data extraction.
FS: screening, full‐text retrieval, data extraction.
HJS: protocol development, data interpretation, methodologic expertise.
EAA: protocol development, data analysis, data interpretation, manuscript drafting, methodologic expertise, review co‐ordination.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
National Institute for Health Research Cochrane Review Incentive Scheme 2016. Award reference Number 16/72/24, UK.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the
Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group -
American Society of Hematology, USA.
This project was supported by the American Society of Hematology
Declarations of interest
LAK: declares no conflicts of interests.
IGT: declares no conflicts of interests.
MBH: declares no conflicts of interests.
CM: declares no conflicts of interests.
MB: declares no conflicts of interests.
VY: declares no conflicts of interests.
IT: declares no conflicts of interests.
FS: declares no conflicts of interests.
HJS: panel member of the ASH VTE in cancer patients, Vice‐Chair of the ASH VTE guidelines and played various leadership roles from 1999 until 2014 with ACCP VTE guidelines.
EAA: served on the executive committee the ACCP Antithrombotic Therapy Guidelines published in 2016.
Acknowledgements
We would like to thank Ms Annie Young, Dr Abderrahman Abdelkefi, Dr Murray Bern, Dr Alison Inder, Dr Patrick Mismetti, and Dr Manuel Monreal for supplying us with the requested information to complete this review. We thank Ms Ann Grifasi for her administrative support. We also thank Dr Assem Khamis for his help with conducting the sensitivity analysis. We thank Dr Elie Ramly and Dr Deborah Cook for their contributions to previous versions of this systematic review
We thank Jo Morrison, Co‐ordinating Editor for the Cochrane Gynaecological Neuro‐oncology and Orphan Cancers Group. We also thank Gail Quinn, Managing Editor of the Cochrane Gynaecological Neuro‐oncology and Orphan Cancers Group for her exceptional support. We thank Joanne Platt, the information specialist of the Cochrane Gynaecological Neuro‐oncology and Orphan Cancers Group, for setting up and managing the monthly alerts.
As described under “Sources of Support” this update was supported in part by the American Society of Hematology to inform ASH guidelines on the topic. We thank the ASH guideline panel for prioritizing questions previously addressed by our review and for critically reviewing our work, including Drs. Pablo Alonso, Waleed Alhazanni, Marc Carrier, Cihan Ay, Marcello DiNisio, Lisa Hicks, Alok Khorana, Andrew Leavitt, Agnes Lee, Gary Lyman, Fergus Macbeth, Rebecca Morgan, Simon Noble, and David Stenehjem and patient representatives Jackie Cook and Elizabeth Sexton. Their input was valuable in validating some of the review related decisions such as eligibility of included studies and the analytical approach.
For our update of these reviews, we followed Cochrane methods using the same eligibility criteria and outcomes used previously. The ASH guidelines group used slightly different methods that generated slightly different results. For example, the ASH guideline panel agreed to prioritize different outcomes; include unpublished data; include abstracts; use different definitions for duration of treatment; and rate certainty of evidence slightly differently for some outcomes, for instance because of imprecision or indirectness. These differences are not described in this publication. Instead, they will be described in the ASH guideline publication.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service, or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Jun 01 | Anticoagulation for people with cancer and central venous catheters | Review | Lara A Kahale, Ibrahim G Tsolakian, Maram B Hakoum, Charbel F Matar, Maddalena Barba, Victor ED Yosuico, Irene Terrenato, Francesca Sperati, Holger Schünemann, Elie A Akl | |
| 2014 Oct 15 | Anticoagulation for people with cancer and central venous catheters | Review | Elie A Akl, Elie P Ramly, Lara A Kahale, Victor E D Yosuico, Maddalena Barba, Francesca Sperati, Deborah Cook, Holger Schünemann | |
| 2011 Apr 13 | Anticoagulation for patients with cancer and central venous catheters | Review | Elie A Akl, Srinivasa Rao Vasireddi, Sameer Gunukula, Victor E D Yosuico, Maddalena Barba, Francesca Sperati, Deborah Cook, Holger Schünemann | |
| 2011 Feb 16 | Anticoagulation for patients with cancer and central venous catheters | Review | Elie A Akl, Srinivasa Rao Vasireddi, Sameer Gunukula, Victor E D Yosuico, Maddalena Barba, Francesca Sperati, Deborah Cook, Holger Schünemann | |
| 2007 Jul 18 | Anticoagulation for thrombosis prophylaxis in cancer patients with central venous catheters | Review | Elie A Akl, Ganesh Kamath, Victor E D Yosuico, Seo Young Kim, Maddalena Barba, Francesca Sperati, Deborah Cook, Holger Schünemann | |
| 2007 Apr 18 | Anticoagulation for thrombosis prophylaxis in cancer patients with central venous lines | Protocol | Elie A Akl, Ganesh Kamath, Holger Schünemann, Maddalena Barba, Y S Kim, Victor E D Yosuico, Deborah Cook, Francesca Sperati, Seo Young Kim | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Anticoagulants [adverse effects, *therapeutic use];
- Catheter-Related Infections [epidemiology];
- Catheterization, Central Venous [*adverse effects];
- Heparin [adverse effects, therapeutic use];
- Heparin, Low-Molecular-Weight [adverse effects, therapeutic use];
- Neoplasms [mortality, *therapy];
- Randomized Controlled Trials as Topic;
- Secondary Prevention [methods];
- Thrombocytopenia [chemically induced];
- Venous Thrombosis [etiology, mortality, *prevention & control];
- Vitamin K [antagonists & inhibitors];
Medical Subject Headings Check Words
Adult; Child; Humans;
PICOs

Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
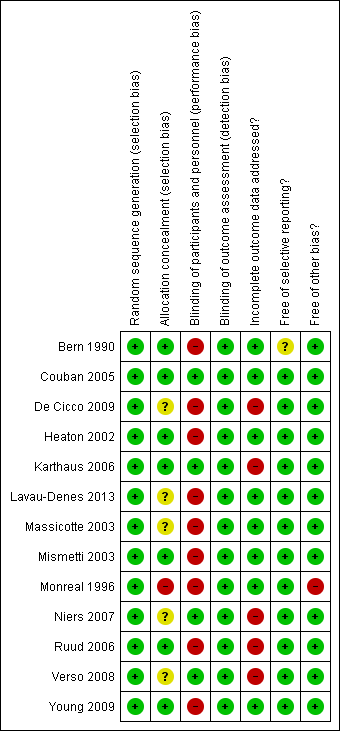
Risk of bias summary: review authors' judgments about each risk of bias item for each included study.

Comparison 1 Low‐molecular‐weight heparin (LMWH) versus no LMWH, Outcome 1 All‐cause mortality (up to 3 months).

Comparison 1 Low‐molecular‐weight heparin (LMWH) versus no LMWH, Outcome 2 Symptomatic catheter‐related thrombosis (up to 3 months).

Comparison 1 Low‐molecular‐weight heparin (LMWH) versus no LMWH, Outcome 3 Asymptomatic catheter‐related thrombosis (up to 3 months).

Comparison 1 Low‐molecular‐weight heparin (LMWH) versus no LMWH, Outcome 4 Major bleeding (up to 3 months).

Comparison 1 Low‐molecular‐weight heparin (LMWH) versus no LMWH, Outcome 5 Minor bleeding (up to 3 months).

Comparison 1 Low‐molecular‐weight heparin (LMWH) versus no LMWH, Outcome 6 Catheter‐related infection (up to 3 months).

Comparison 1 Low‐molecular‐weight heparin (LMWH) versus no LMWH, Outcome 7 Thrombocytopenia (up to 3 months).
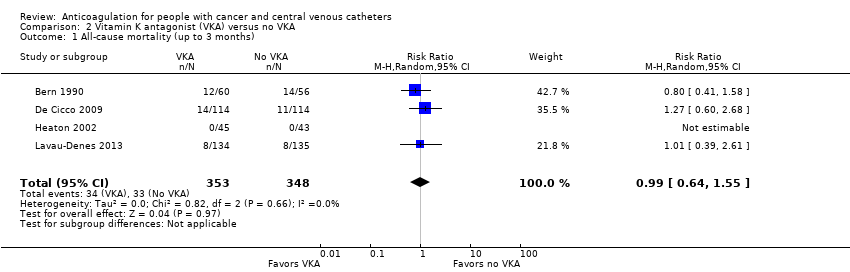
Comparison 2 Vitamin K antagonist (VKA) versus no VKA, Outcome 1 All‐cause mortality (up to 3 months).

Comparison 2 Vitamin K antagonist (VKA) versus no VKA, Outcome 2 Symptomatic catheter‐related thrombosis (up to 3 months).

Comparison 2 Vitamin K antagonist (VKA) versus no VKA, Outcome 3 Asymptomatic catheter‐related thrombosis.

Comparison 2 Vitamin K antagonist (VKA) versus no VKA, Outcome 4 Major bleeding (up to 3 months).
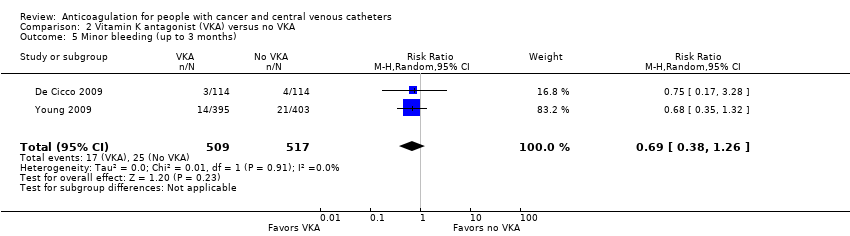
Comparison 2 Vitamin K antagonist (VKA) versus no VKA, Outcome 5 Minor bleeding (up to 3 months).
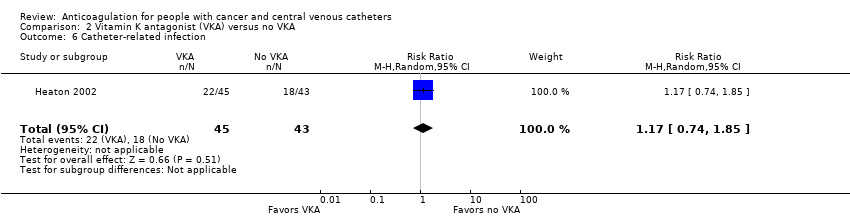
Comparison 2 Vitamin K antagonist (VKA) versus no VKA, Outcome 6 Catheter‐related infection.

Comparison 2 Vitamin K antagonist (VKA) versus no VKA, Outcome 7 Premature central venous catheter removal.

Comparison 3 Low‐molecular‐weight heparin (LMWH) versus vitamin K antagonist (VKA), Outcome 1 All‐cause mortality (up to 3 months).
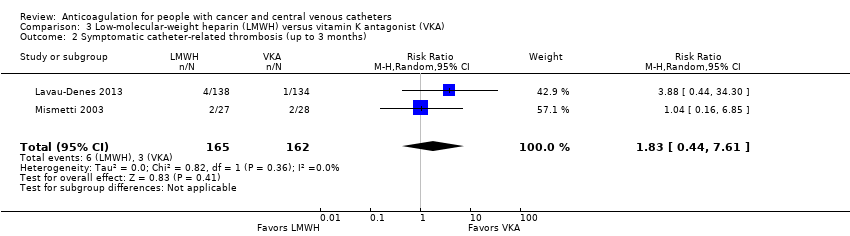
Comparison 3 Low‐molecular‐weight heparin (LMWH) versus vitamin K antagonist (VKA), Outcome 2 Symptomatic catheter‐related thrombosis (up to 3 months).

Comparison 3 Low‐molecular‐weight heparin (LMWH) versus vitamin K antagonist (VKA), Outcome 3 Asymptomatic catheter‐related thrombosis.

Comparison 3 Low‐molecular‐weight heparin (LMWH) versus vitamin K antagonist (VKA), Outcome 4 Pulmonary embolism (up to 3 months).
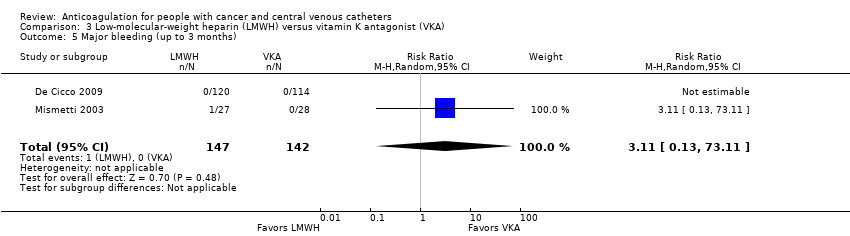
Comparison 3 Low‐molecular‐weight heparin (LMWH) versus vitamin K antagonist (VKA), Outcome 5 Major bleeding (up to 3 months).

Comparison 3 Low‐molecular‐weight heparin (LMWH) versus vitamin K antagonist (VKA), Outcome 6 Minor bleeding (up to 3 months).

Comparison 3 Low‐molecular‐weight heparin (LMWH) versus vitamin K antagonist (VKA), Outcome 7 Thrombocytopenia (up to 3 months).
| Low‐molecular‐weight heparin (LMWH) compared to no LMWH for people with cancer and central venous catheters | |||||
| Patient or population: people with cancer with thrombosis prophylaxis and central venous catheters Settings: outpatient or inpatient Intervention: LMWH Comparison: no LMWH | |||||
| Outcomes (follow‐up) | № of participants | Certainty of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with no LMWH | Risk difference with LMWH | ||||
| All‐cause mortality (up to 3 months) | 1236 | ⊕⊕⊝⊝ | RR 0.82 | Study population | |
| 77 per 1000 | 14 fewer per 1000 | ||||
| Symptomatic catheter‐related thrombosis (up to 3 months) | 1089 | ⊕⊕⊕⊝ | RR 0.43 | Study population | |
| 67 per 1000 | 38 fewer per 1000 | ||||
| Symptomatic catheter‐related thrombosis measured as asymptomatic catheter‐related thrombosis (up to 3 months) | 1089 | ⊕⊝⊝⊝ | RR 0.95 | Study population | |
| 96 per 1000 | 5 fewer per 1000 | ||||
| Major bleeding (up to 3 months) | 1018 | ⊕⊝⊝⊝ | RR 1.49 | Study population | |
| 0 per 1000 | 0 fewer per 1000 | ||||
| Low | |||||
| 1 per 1000 | 0 fewer per 1000 | ||||
| Minor bleeding (up to 3 months) | 544 | ⊕⊕⊝⊝ | RR 1.35 | Study population | |
| 41 per 1000 | 14 more per 1000 | ||||
| Catheter‐related infection (up to 3 months) | 474 | ⊕⊕⊝⊝ | RR 0.97 | Study population | |
| 92 per 1000 | 3 fewer per 1000 | ||||
| Thrombocytopenia (up to 3 months) | 1002 | ⊕⊕⊝⊝ | RR 1.03 | Study population | |
| 176 per 1000 | 5 more per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; LMWH: low‐molecular‐weight heparin; RCT: randomized controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded by one level due to serious risk of bias; lack of blinding in participants and personnel in three studies, incomplete outcome data not addressed in three studies, and unclear or no allocation concealment in four out of five studies. bDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (36 per 1000 absolute reduction) and the possibility of important harm (20 per 1000 absolute increase), including 79 events in total. cDowngraded by one level due to serious risk of bias; lack of blinding in participants and personnel in two studies; incomplete outcome data not addressed in three studies; and unclear or no allocation concealment in four out of five studies. dDowngraded by one level due to concern about inconsistency, outcome measured as surrogate outcome. eDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (36 per 1000 absolute reduction) and the possibility of important harm (40 per 1000 absolute increase), including 94 events in total. fDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in two studies; incomplete outcome data not addressed in three studies; and unclear or no allocation concealment in four out of five studies. gDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in one study; incomplete outcome data not addressed in four studies; and unclear or no allocation concealment in three out of four studies. hDowngraded by two levels due to concern about imprecision; 95% CI was consistent with the possibility for benefit (1 per 1000 absolute reduction) and the possibility of important harm (35 per 1000 absolute increase), including five events in total. Given the observed baseline risk of 0% we used 0.1% to generate an absolute effect and a confidence interval. iDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in one study; incomplete outcome data not addressed in two studies; and unclear or no allocation concealment in two out of two studies. jDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (16 per 1000 absolute reduction) and the possibility of important harm (79 per 1000 absolute increase), including 26 events in total. kDowngraded by one level due to concern about risk of bias; incomplete outcome data not addressed in two studies; and unclear or no allocation concealment in one out of two studies. lDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (35 per 1000 absolute reduction) and the possibility of important harm (58 per 1000 absolute increase), including 36 events in total. mDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in two studies; incomplete outcome data not addressed in two studies; and unclear or no allocation concealment in three out of four studies. nDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (35 per 1000 absolute reduction) and the possibility of important harm (58 per 1000 absolute increase) including 163 events in total. | |||||
| Vitamin K antagonist (VKA) compared to no VKA for people with cancer and central venous catheters | |||||
| Patient or population: people with cancer with thrombosis prophylaxis and central venous catheters Settings: outpatient or inpatient Intervention: VKA Comparison: no VKA | |||||
| Outcomes (follow‐up) | № of participants | Certainty of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with no VKA | Risk difference with VKA | ||||
| All‐cause mortality (up to 3 months) | 701 | ⊕⊕⊝⊝ | RR 0.99 | Study population | |
| 95 per 1000 | 1 fewer per 1000 | ||||
| Symptomatic catheter‐related thrombosis (up to 3 months) | 1271 | ⊕⊕⊝⊝ | RR 0.61 | Study population | |
| 80 per 1000 | 31 fewer per 1000 | ||||
| Symptomatic catheter‐related thrombosis measured as asymptomatic catheter‐related thrombosis (up to 3 months) | 384 | ⊕⊝⊝⊝ | RR 0.61 | Study population | |
| 73 per 1000 | 29 fewer per 1000 | ||||
| Major bleeding (up to 3 months) | 1026 | ⊕⊕⊝⊝ | RR 7.14 | Study population | |
| 2 per 1000 | 12 more per 1000 | ||||
| Minor bleeding (up to 3 months) | 1026 | ⊕⊕⊝⊝ | RR 0.69 | Study population | |
| 48 per 1000 | 15 fewer per 1000 | ||||
| Catheter‐related infection | 88 | ⊕⊕⊝⊝ | RR 1.17 | Study population | |
| 419 per 1000 | 71 more per 1000 | ||||
| Premature CVC removal | 88 | ⊕⊕⊝⊝ | RR 0.82 | Study population | |
| 163 per 1000 | 29 fewer per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CVC: central venous catheter; RCT: randomized controlled trial; RR: risk ratio; VKA: vitamin K antagonist. | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded by one level due to concern about risk of bias (lack of blinding in participants and personnel in four studies and unclear or no allocation concealment in two out of four studies. bDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (34 per 1000 absolute reduction) and the possibility of important harm (52 per 1000 absolute increase), including 67 events in total. cThe trial WARP showed no overall survival advantage in participants taking warfarin compared with participants in the no‐warfarin group (hazard ratio 0.98, 95% CI 0.77 to 1.25; P = 0.26) (Young 2009). dDowngraded by one level due to concern about risk of bias (lack of blinding in participants and personnel in four studies and no clear information concerning allocation concealment in one out of four studies). eDowngraded by one level due to unexplained inconsistency (I2 = 70%). Imprecision was partially driven by the inconsistency between the studies and was taken into consideration when downgrading by two levels for serious risk of bias and serious inconsistency. fDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (63 per 1000 absolute reduction) and the possibility of important harm (57 per 1000 absolute increase), including 87 events in total. gDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in two studies and no clear information about allocation concealment in one out of two studies. hDowngraded by one level due to concern about inconsistency, outcome measured as surrogate outcome. iDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (54 per 1000 absolute reduction) and the possibility of important harm (29 per 1000 absolute increase), including 22 events in total. jDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in two studies and no clear information about allocation concealment in one out of two studies. kDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for no effect (0 per 1000 absolute reduction) and the possibility of important harm (120 per 1000 absolute increase), including eight events in total. lDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in three studies; and unclear or no allocation concealment in two out of three studies. mDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (30 per 1000 absolute reduction) and the possibility of important harm (16 per 1000 absolute increase), including 42 events in total. nDowngraded by one level due to concern about risk of bias (lack of blinding in participants and personnel in the included study). oDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (109 per 1000 absolute reduction) and the possibility of important harm (356 per 1000 absolute increase), including 40 events in total. pDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (114 per 1000 absolute reduction) and the possibility of important harm (202 per 1000 absolute increase), including 13 events in total. | |||||
| Low‐molecular‐weight heparin (LMWH) compared to vitamin K antagonist (VKA) for people with cancer and central venous catheters | |||||
| Patient or population: people with cancer with thrombosis prophylaxis and central venous catheters Settings: outpatient or inpatient Intervention: LMWH Comparison: VKA | |||||
| Outcomes (follow‐up) | № of participants | Certainty of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with VKA | Risk difference with LMWH | ||||
| All‐cause mortality (up to 3 months) | 561 | ⊕⊕⊝⊝ | RR 0.94 | Study population | |
| 94 per 1000 | 6 fewer per 1000 | ||||
| Symptomatic catheter‐related thrombosis (up to 3 months) | 327 | ⊕⊝⊝⊝ | RR 1.83 | Study population | |
| 19 per 1000 | 15 more per 1000 | ||||
| Symptomatic catheter‐related thrombosis measured as asymptomatic catheter‐related thrombosis (up to 3 months) | 317 | ⊕⊝⊝⊝ | RR 1.61 | Study population | |
| 63 per 1000 | 39 more per 1000 | ||||
| Pulmonary embolism (up to 3 months) | 327 | ⊕⊕⊝⊝ | RR 1.70 | Study population | |
| 49 per 1000 | 35 more per 1000 | ||||
| Major bleeding (up to 3 months) | 289 | ⊕⊝⊝⊝ | RR 3.11 | Study population | |
| 0 per 1000 | 0 fewer per 1000 | ||||
| Low | |||||
| 1 per 1000 | 2 more per 1000 | ||||
| Minor bleeding (up to 3 months) | 234 | ⊕⊝⊝⊝ | RR 0.95 | Study population | |
| 26 per 1000 | 1 fewer per 1000 | ||||
| Thrombocytopenia (up to 3 months)n | 327 | ⊕⊕⊕⊝ | RR 1.69 | Study population | |
| 216 per 1000 | 149 more per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; LMWH: low‐molecular‐weight heparin; RCT: randomized controlled trial; RR: risk ratio; VKA: vitamin K antagonist. | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in three studies; and unclear allocation concealment in two out of three studies. bDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (41 per 1000 absolute reduction) and the possibility of important harm (56 per 1000 absolute increase), including 51 events in total. cDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in two studies and unclear allocation concealment in one out of two studies. dDowngraded by two levels due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (10 per 1000 absolute reduction) and the possibility of important harm (122 per 1000 absolute increase), including nine events in total. eDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in two studies; and unclear allocation concealment in one out of two studies. fDowngraded by one level due to concern about inconsistency, outcome measured as surrogate outcome. gDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (16 per 1000 absolute reduction) and the possibility of important harm (156 per 1000 absolute increase), including 26 events in total. hDowngraded by one level due to concern both risk of bias; lack of blinding in participants and personnel in two studies; and allocation concealment not clear in one out of two studies. iDowngraded by one level due to concern about imprecision; 95% CI was consistent with the possibility for important benefit (13 per 1000 absolute reduction) and the possibility of important harm (144 per 1000 absolute increase), including 22 events in total. jDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in two studies; and allocation concealment not clear in one out of two studies. kDowngraded by two levels due to concern about imprecision; 95% CI was consistent with the possibility for benefit (1 per 1000 absolute reduction) and the possibility of important harm (51 per 1000 absolute increase), including one event in total. Given the observed baseline risk of 0% we used 0.1% to generate an absolute effect and a confidence interval. lDowngraded by one level due to concern about risk of bias (lack of blinding in participants and personnel in the study and unclear allocation concealment). mDowngraded by two levels due to concern about imprecision (95% CI was consistent with the possibility for benefit (21 per 1000 absolute reduction) and the possibility of important harm (95 per 1000 absolute increase), including six events in total. nThe study by Lavau‐Denes and colleagues included all grades of thrombocytopenia (even mild cases) (Lavau‐Denes 2013). oDowngraded by one level due to concern about risk of bias; lack of blinding in participants and personnel in two studies; and allocation concealment not clear in one out of two studies. | |||||
| Term | Definition |
| Adjuvant therapy | Assisting in the amelioration, or cure of disease |
| Anticoagulation | Process of hindering the clotting of blood especially by treatment with an anticoagulant. |
| Antithrombotic | Used against or tending to prevent thrombosis (clotting) |
| Bacteremia | Presence of bacteria in the blood |
| Central venous line | Synthetic tube that is inserted into a central (large) vein to provide temporary intravenous access for the administration of fluid, medication, or nutrients. |
| Coagulation | Clotting |
| Deep venous (vein) thrombosis (DVT) | Condition marked by the formation of a thrombus within a deep vein (as of the leg or pelvis) that may be asymptomatic or be accompanied by symptoms (as swelling and pain) and that is potentially life threatening if dislodgment of the thrombus results in pulmonary embolism |
| Fibrin | White insoluble fibrous protein formed from fibrinogen by the action of thrombin especially in the clotting of blood |
| Fondaparinux | Anticoagulant medication |
| Hemostatic system | System that shortens the clotting time of blood and stops bleeding |
| Heparin | Enzyme occurring especially in the liver and lungs that prolongs the clotting time of blood by preventing the formation of fibrin. 2 forms of heparin that are used as anticoagulant medications are: unfractionated heparin (UFH) and low‐molecular‐weight heparin (LMWH) |
| Impedance plethysmography | Technique that measures the change in blood volume (venous blood volume as well as the pulsation of the arteries) for a specific body segment |
| Kappa statistics | Measure of degree of non‐random agreement between observers or measurements of a specific categorical variable or both |
| Metastasis | Spread of cancer cells from the initial or primary site of disease to another part of the body |
| Oncogene | Gene having the potential to cause a normal cell to become cancerous |
| Osteoporosis | Condition that affects especially older women and is characterized by decrease in bone mass with decreased density and enlargement of bone spaces producing porosity and brittleness |
| Parenteral nutrition | Practice of feeding a person intravenously, circumventing the gastrointestinal tract |
| Pulmonary embolism (PE) | Embolism of a pulmonary artery or 1 of its branches that is produced by foreign matter and most often a blood clot originating in a vein of the leg or pelvis and that is marked by labored breathing, chest pain, fainting, rapid heart rate, cyanosis, shock, and sometimes death. |
| Stroma | Supporting framework of an organ typically consisting of connective tissue |
| Thrombin | Proteolytic enzyme formed from prothrombin that facilitates the clotting of blood by catalyzing conversion of fibrinogen to fibrin |
| Thrombocytopenia | Persistent decrease in the number of blood platelets that is often associated with hemorrhagic conditions |
| Thrombosis | Formation or presence of a blood clot within a blood vessel |
| Vitamin K antagonists (VKA) | Anticoagulant medications that are used for anticoagulation. Warfarin is a vitamin K antagonist |
| Warfarin | Anticoagulant medication that is a vitamin K antagonist that is used for anticoagulation |
| Ximelagatran | Anticoagulant medication |
| LMWH | Generic name | Prophylactic dose | Therapeutic dose |
| Lovenox | Enoxaparin | 40 mg once daily | 1 mg/kg twice daily |
| Fragmin | Dalteparin | 2500‐5000 U once daily | 200 U/kg once daily or |
| Innohep | Tinzaparin, logiparin | 4500 U once daily | 90 U/kg twice daily |
| Fraxiparine | Nadroparin | 35‐75 anti‐Xa IU/kg/day | 175 anti‐Xa IU/kg/day |
| Certoparin | Sandoparin | 3000 anti‐Xa IU once daily | – |
| Reviparin | Reviparin | 1750‐4200 anti‐Xa IU | 7000‐12,600 anti‐Xa IU |
| IU: international units; U: units; Xa: factor Xa. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality (up to 3 months) Show forest plot | 5 | 1236 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.53, 1.26] |
| 2 Symptomatic catheter‐related thrombosis (up to 3 months) Show forest plot | 5 | 1089 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.22, 0.81] |
| 3 Asymptomatic catheter‐related thrombosis (up to 3 months) Show forest plot | 5 | 1089 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.62, 1.46] |
| 4 Major bleeding (up to 3 months) Show forest plot | 4 | 1018 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.06, 36.28] |
| 5 Minor bleeding (up to 3 months) Show forest plot | 2 | 544 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.62, 2.92] |
| 6 Catheter‐related infection (up to 3 months) Show forest plot | 2 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.52, 1.79] |
| 7 Thrombocytopenia (up to 3 months) Show forest plot | 4 | 1002 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.80, 1.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality (up to 3 months) Show forest plot | 4 | 701 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.64, 1.55] |
| 2 Symptomatic catheter‐related thrombosis (up to 3 months) Show forest plot | 4 | 1271 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.23, 1.64] |
| 3 Asymptomatic catheter‐related thrombosis Show forest plot | 2 | 384 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.27, 1.40] |
| 4 Major bleeding (up to 3 months) Show forest plot | 2 | 1026 | Risk Ratio (M‐H, Random, 95% CI) | 7.14 [0.88, 57.78] |
| 5 Minor bleeding (up to 3 months) Show forest plot | 2 | 1026 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.38, 1.26] |
| 6 Catheter‐related infection Show forest plot | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.74, 1.85] |
| 7 Premature central venous catheter removal Show forest plot | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.30, 2.24] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality (up to 3 months) Show forest plot | 3 | 561 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.56, 1.59] |
| 2 Symptomatic catheter‐related thrombosis (up to 3 months) Show forest plot | 2 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [0.44, 7.61] |
| 3 Asymptomatic catheter‐related thrombosis Show forest plot | 2 | 317 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.75, 3.46] |
| 4 Pulmonary embolism (up to 3 months) Show forest plot | 2 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [0.74, 3.92] |
| 5 Major bleeding (up to 3 months) Show forest plot | 2 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 3.11 [0.13, 73.11] |
| 6 Minor bleeding (up to 3 months) Show forest plot | 1 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.20, 4.61] |
| 7 Thrombocytopenia (up to 3 months) Show forest plot | 2 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [1.20, 2.39] |


