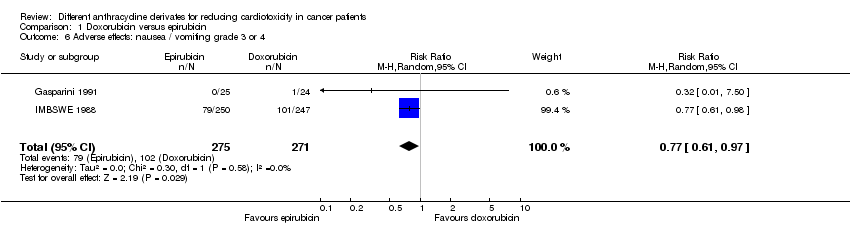
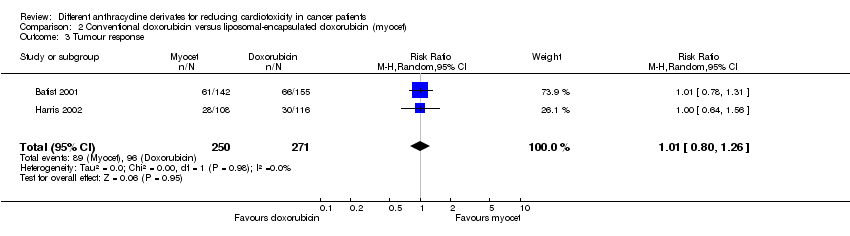
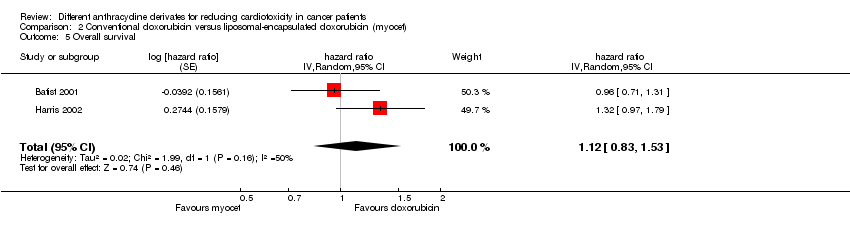
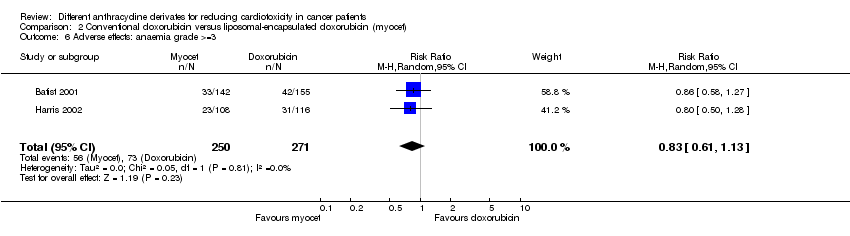
Related content
Related reviews and protocols
Elvira C van Dalen, Erna MC Michiels, Huib N Caron, Leontien CM Kremer | 12 May 2010
Elvira C van Dalen, Helena JH van der Pal, Leontien CM Kremer | 3 March 2016
Elvira C van Dalen, Huib N Caron, Heather O Dickinson, Leontien CM Kremer | 15 June 2011
Lesley A Smith, Fredric Azariah, Verna TC Lavender, Nicola S Stoner, Silvana Bettiol | 12 November 2015
Chemoradiotherapy for Cervical Cancer Meta‐analysis Collaboration (CCCMAC) | 20 January 2010
Liat Vidal, Itsik Ben dor, Mical Paul, Noa Eliakim‐Raz, Ellisheva Pokroy, Karla Soares‐Weiser, Leonard Leibovici | 9 October 2013
Khadra Galaal, Mansour Al Moundhri, Andrew Bryant, Alberto D Lopes, Theresa A Lawrie | 15 May 2014
Esmée C de Baat, Renée L Mulder, Saro Armenian, Elizabeth AM Feijen, Heynric Grotenhuis, Melissa M Hudson, Annelies MC Mavinkurve-Groothuis, Leontien CM Kremer, Elvira C van Dalen | 27 September 2022
Kenneth Jaaback, Nick Johnson, Theresa A Lawrie | 12 January 2016
Yunhai Chuaia, Ivana Rizzutoa, Xia Zhanga, Ying Li, Guanghai Dai, Sophie J Otter, Rasiah Bharathan, Alexandra Stewart, Aiming Wang | 4 March 2021