Ejercicios para mujeres que reciben tratamiento adyuvante para el cáncer de mama
Information
- DOI:
- https://doi.org/10.1002/14651858.CD005001.pub3Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 21 September 2016see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Breast Cancer Group
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
ACF: handsearching and screening search results, study selection, data extraction, contacted experts for unpublished trials and trial investigators for additional data, methodological assessments, quantitative and qualitative synthesis of included studies, reporting.
MM: screening search results, study selection, methodological assessments, contributed to consensus finding when disagreement in study selection, data extraction or methodological assessments persisted between the other two review authors (ACF, MHM), quantitative and qualitative synthesis of included studies, manuscript review.
MHM: handsearching and screening search results, study selection, data extraction, methodological assessments, contributed to consensus finding when disagreement in study selection or methodological assessments persisted between the other two review authors (ACF, MM), quantitative and qualitative synthesis of included studies, reporting.
Declarations of interest
ACF, MM, MHM: None known.
Acknowledgements
We would like to thank Melina Wilson, who rendered assistance in her role as Managing Editor of the Cochrane Breast Cancer Group and the Editorial Base of the Cochrane Breast Cancer Group. Moreover, we would like to thank the referees who provided valuable feedback during the peer review process and the authors of primary trials for additional information about their trials. Anke Schulz rendered support in statistics for this update version of the review. Juyoung Byun from the Korean Branch of the Australasian Cochrane Centre assisted with one Korean language publication. We also thank Karl‐Ludwig Resch for obtaining several dissertations and for help with data extraction and 'Risk of bias' assessment of one trial.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Sep 21 | Exercise for women receiving adjuvant therapy for breast cancer | Review | Anna C Furmaniak, Matthias Menig, Martina H Markes | |
| 2006 Oct 18 | Exercise for women receiving adjuvant therapy for breast cancer | Review | Martina Markes, Thomas Brockow, Karl‐Ludwig Resch | |
| 2004 Oct 18 | Exercises for women receiving adjuvant therapy for breast cancer | Protocol | Martina Markes, Thomas Brockow, Karl‐Ludwig Resch | |
Differences between protocol and review
We included only randomised controlled trials in this updated version of the review, as opposed to the original version of the review, in which we also included controlled trials without a randomisation procedure. This was due to the increasing number of randomised trials.
We excluded hormonal therapy only as adjuvant therapy in this version of the review as we did not consider the side effects of hormonal therapy comparable in their severity to chemo‐ or radiotherapy or both. We did not include biological outcomes like immune function measured with T‐cells in this version of the review, as the focus of the review is on directly participant‐relevant outcomes. The same applied to morphological outcomes (changes in body weight or body composition).
We classified outcomes into primary and secondary outcomes in this version of the review.
We did not include data for outcomes assessed with subscales of questionnaires (for example physical functioning subscale of the SF‐36 or vitality subscale of the SF‐36, nausea item of the SCL‐90) in this version of the review.
We also assessed the quality of evidence for the main outcomes using the GRADE methodology and developed a 'Summary of findings' table. This ensures that the review complies with new Cochrane methodological standards.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Breast Neoplasms [psychology, *therapy];
- Chemotherapy, Adjuvant [adverse effects];
- Cognition;
- Depression [therapy];
- *Exercise Therapy;
- Fatigue [rehabilitation];
- Lymphedema [etiology];
- Physical Fitness;
- Quality of Life;
- Radiotherapy, Adjuvant [adverse effects];
- Randomized Controlled Trials as Topic;
- Weight Gain;
Medical Subject Headings Check Words
Female; Humans;
PICOs

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
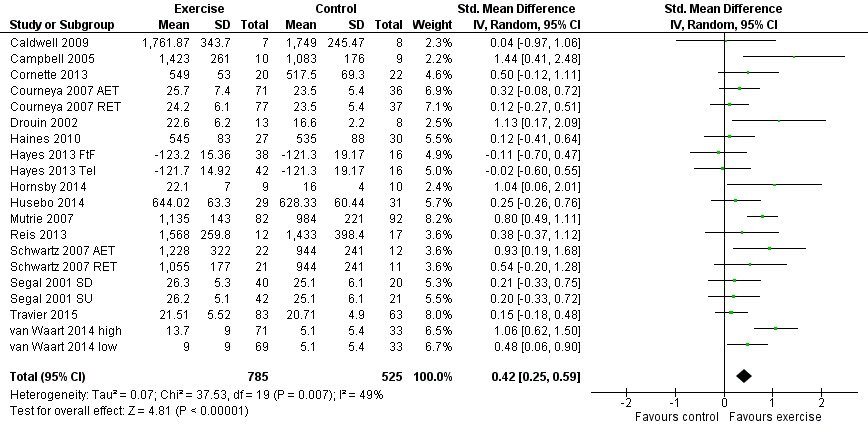
Forest plot of comparison: 1 Exercise versus control, outcome: 1.1 Physical fitness.

Forest plot of comparison: 1 Exercise versus control, outcome: 1.2 Fatigue.
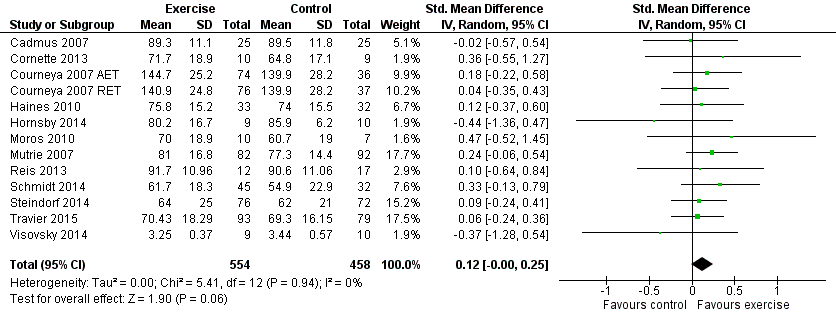
Forest plot of comparison: 1 Exercise versus control, outcome: 1.3 Cancer‐specific quality of life.

Forest plot of comparison: 1 Exercise versus control, outcome: 1.6 Depression.

Comparison 1 Exercise versus control, Outcome 1 Physical fitness.

Comparison 1 Exercise versus control, Outcome 2 Fatigue.

Comparison 1 Exercise versus control, Outcome 3 Cancer‐specific quality of life.

Comparison 1 Exercise versus control, Outcome 4 Health‐related quality of life.

Comparison 1 Exercise versus control, Outcome 5 Cancer site‐specific quality of life.

Comparison 1 Exercise versus control, Outcome 6 Depression.

Comparison 1 Exercise versus control, Outcome 7 Cognitive function.

Comparison 1 Exercise versus control, Outcome 8 Strength.

Comparison 1 Exercise versus control, Outcome 9 Subjective upper body function.

Comparison 1 Exercise versus control, Outcome 10 Shoulder mobility.

Comparison 1 Exercise versus control, Outcome 11 Arm morbidity.

Comparison 1 Exercise versus control, Outcome 12 Anxiety.

Comparison 1 Exercise versus control, Outcome 13 Mood disturbances.

Comparison 1 Exercise versus control, Outcome 14 Hospital Anxiety and Depression Scale.

Comparison 1 Exercise versus control, Outcome 15 Self esteem.

Comparison 1 Exercise versus control, Outcome 16 Physical activity.

Comparison 1 Exercise versus control, Outcome 17 Neuropathic pain.

Comparison 1 Exercise versus control, Outcome 18 Neuropathy symptoms.

Comparison 1 Exercise versus control, Outcome 19 Endocrine symptoms.

Comparison 1 Exercise versus control, Outcome 20 Gait and balance.

Comparison 1 Exercise versus control, Outcome 21 Lymphoedema incidence.

Comparison 2 Exercise versus control follow‐up, Outcome 1 Physical fitness.

Comparison 2 Exercise versus control follow‐up, Outcome 2 Fatigue.

Comparison 2 Exercise versus control follow‐up, Outcome 3 Cancer‐specific quality of life.

Comparison 2 Exercise versus control follow‐up, Outcome 4 Depression.

Comparison 2 Exercise versus control follow‐up, Outcome 5 Strength.

Comparison 2 Exercise versus control follow‐up, Outcome 6 Physical activity.

Comparison 2 Exercise versus control follow‐up, Outcome 7 Anxiety.

Comparison 2 Exercise versus control follow‐up, Outcome 8 Self esteem.

Comparison 2 Exercise versus control follow‐up, Outcome 9 Endocrine symptoms.

Comparison 2 Exercise versus control follow‐up, Outcome 10 Neuropathy symptoms.

Comparison 2 Exercise versus control follow‐up, Outcome 11 Gait and balance.

Comparison 2 Exercise versus control follow‐up, Outcome 12 Lymphoedema incidence.
| Exercise compared with control for women receiving adjuvant therapy for breast cancer | ||||
| Population: women receiving adjuvant therapy (chemo‐ or radiotherapy or both) for breast cancer Settings: supervised or home based Intervention: aerobic or resistance exercise or a combination of both Comparison: control intervention (usual care or intervention that was not exercise, such as stretching) | ||||
| Outcomes | Relative effects* (95% CI) | No of Participants | Quality of the evidence | Comments |
| Exercise vs control | ||||
| Physical fitness assessed with: 6‐ or 12‐minute walk test, peak oxygen uptake, and other scales (follow‐up: 18 weeks to 6 months) | The mean physical fitness in the intervention group was 0.42 standard deviations higher (0.25 to 0.59 higher) | 1310 (15 RCTs) | ⊕⊕⊕⊝ | SMD 0.42 (95% CI 0.25 to 0.59) |
| Fatigue assessed with: FACIT‐F scale, (revised) Piper Fatigue Scale, Multidimensional Fatigue Inventory and other scales (follow‐up: 18 weeks to 6 months) | The mean fatigue in the intervention group was 0.28 standard deviations lower (0.41 lower to 0.16 lower) | 1698 (19 RCTs) | ⊕⊕⊕⊝ | SMD ‐0.28 (95% CI ‐0.41 to ‐0.16) |
| Cancer‐specific quality of life assessed with: FACT‐G, EORTC QLQ‐C30 and other scales (follow‐up: 12 weeks to 6 months) | The mean cancer‐specific quality of life in the intervention group was 0.12 standard deviations higher (0.00 to 0.25 higher) | 1012 (12 RCTs) | ⊕⊕⊕⊝ | SMD 0.12 (95% CI 0.00 to 0.25) |
| Health‐related quality of life assessed with EQ‐5D visual analogue scale (higher scores indicate higher quality of life, score range from 0 to 100) MID: 7 points (follow‐up: end of intervention) | The mean health‐related quality of life in the intervention group was 1.10 points higher (5.28 lower to 7.48 higher) | 68 (1 RCT) | ⊕⊕⊝⊝ | MD 1.10 (95% CI ‐5.28 to 7.48) |
| Cancer site‐specific quality of life assessed with: FACT‐B (higher scores indicate better quality of life, score range from 0 to 144) MID: 7 to 8 points (follow‐up: end of intervention) | The mean cancer site‐specific quality of life in the intervention group was 4.24 points higher (1.81 lower to 10.29 points higher) | 262 (4 RCTs) | ⊕⊕⊝⊝ | MD 4.24 (95% CI ‐1.81 to 10.29) |
| Depression assessed with: BDI, CES‐D (follow‐up: 6 months) | The mean depression in the intervention group was 0.15 standard deviations lower (0.30 lower to 0.01 higher) | 674 (5 RCTs) | ⊕⊕⊕⊝ | SMD ‐0.15 (95% CI ‐0.30 to 0.01) |
| Cognitive function assessed with: Trail Making Test (less time in seconds needed for completing the test means less cognitive dysfunction) (follow‐up: end of intervention) | The mean time needed for completing the test in the intervention group was 11.55 seconds less (22.06 seconds less to 1.05 seconds less) | 213 (2 RCTs) | ⊕⊕⊝⊝ | MD ‐11.55 (95% CI ‐22.06 to ‐1.05) |
| Lymphoedema assessed with: volumetric arm measurements and bioimpedance spectroscopy (follow‐up: 8 weeks) | Assumed risk11: 60 per 1000 (30 to 123) | 436 (2 RCTs) | ⊕⊕⊝⊝ | RR 0.71 (95% CI 0.35 to 1.45) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||
| GRADE Working Group grades of evidence | ||||
| 1Lack of blinding, low adherence and high or unclear contamination, several randomisation and many allocation concealment procedures were unclear, therefore we downgraded by one level. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Physical fitness Show forest plot | 20 | 1310 | Std. Mean Difference (IV, Random, 95% CI) | 0.42 [0.25, 0.59] |
| 2 Fatigue Show forest plot | 22 | 1698 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.41, ‐0.16] |
| 3 Cancer‐specific quality of life Show forest plot | 13 | 1012 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.00, 0.25] |
| 4 Health‐related quality of life Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Cancer site‐specific quality of life Show forest plot | 4 | 262 | Mean Difference (IV, Random, 95% CI) | 4.24 [‐1.81, 10.29] |
| 6 Depression Show forest plot | 6 | 674 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.30, 0.01] |
| 7 Cognitive function Show forest plot | 2 | 213 | Mean Difference (IV, Random, 95% CI) | ‐11.55 [‐22.06, ‐1.05] |
| 8 Strength Show forest plot | 13 | 912 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [0.04, 0.50] |
| 9 Subjective upper body function Show forest plot | 3 | 231 | Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐4.45, 3.41] |
| 10 Shoulder mobility Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11 Arm morbidity Show forest plot | 3 | 240 | Mean Difference (IV, Random, 95% CI) | 1.11 [‐4.07, 6.29] |
| 12 Anxiety Show forest plot | 3 | 331 | Mean Difference (IV, Random, 95% CI) | ‐1.45 [‐4.36, 1.46] |
| 13 Mood disturbances Show forest plot | 3 | 111 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐1.40, ‐0.60] |
| 14 Hospital Anxiety and Depression Scale Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15 Self esteem Show forest plot | 4 | 323 | Mean Difference (IV, Random, 95% CI) | 1.69 [‐0.01, 3.39] |
| 16 Physical activity Show forest plot | 8 | 549 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [0.12, 0.47] |
| 17 Neuropathic pain Show forest plot | 2 | 130 | Mean Difference (IV, Random, 95% CI) | 3.64 [‐1.32, 8.60] |
| 18 Neuropathy symptoms Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 19 Endocrine symptoms Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 20 Gait and balance Show forest plot | 3 | 122 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.25, 0.46] |
| 21 Lymphoedema incidence Show forest plot | 4 | 436 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.35, 1.45] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Physical fitness Show forest plot | 6 | 612 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.06, 0.57] |
| 2 Fatigue Show forest plot | 8 | 814 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.35, ‐0.07] |
| 3 Cancer‐specific quality of life Show forest plot | 6 | 583 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [0.01, 0.35] |
| 4 Depression Show forest plot | 3 | 378 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.48, ‐0.06] |
| 5 Strength Show forest plot | 4 | 386 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.30, 0.30] |
| 6 Physical activity Show forest plot | 3 | 261 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.05, 0.61] |
| 7 Anxiety Show forest plot | 2 | 201 | Mean Difference (IV, Random, 95% CI) | ‐3.61 [‐7.24, 0.03] |
| 8 Self esteem Show forest plot | 2 | 201 | Mean Difference (IV, Random, 95% CI) | 1.20 [‐0.41, 2.81] |
| 9 Endocrine symptoms Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10 Neuropathy symptoms Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11 Gait and balance Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12 Lymphoedema incidence Show forest plot | 2 | 194 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.37, 1.69] |


