| 1 Participants experiencing exacerbations requiring oral steroid treatment Show forest plot | 4 | 4949 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.74, 1.07] |
|
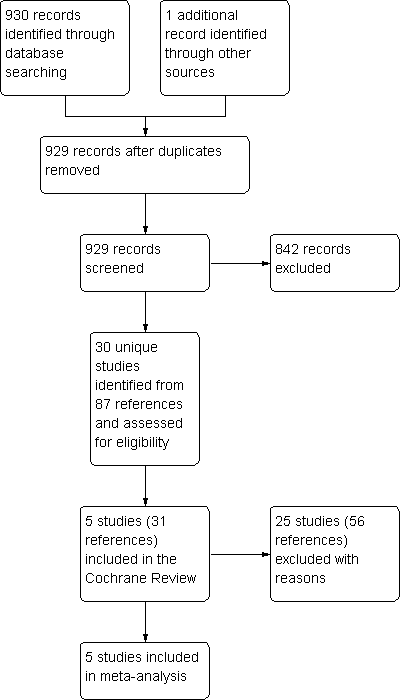
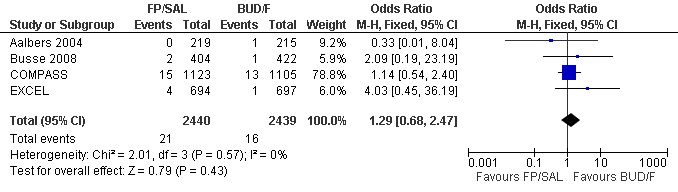
| 2 Participants experiencing exacerbations requiring admission to hospital Show forest plot | 4 | 4879 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.68, 2.47] |
|
| 3 Asthma‐related serious adverse event Show forest plot | 3 | 4054 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.75, 2.86] |
|
| 4 Participants experiencing exacerbations requiring ED visit/hospitalisation Show forest plot | 4 | 4861 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.94, 1.80] |
|
| 5 Mortality Show forest plot | 5 | | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
|
| 6 Asthma Quality of Life Questionnaire Show forest plot | 1 | | Mean Difference (Fixed, 95% CI) | Totals not selected |
|
| 7 N with improvement in Asthma Quality of Life Questionnaire Show forest plot | 1 | | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
|
| 8 Change in am PEF Show forest plot | 5 | 5101 | L/min (Fixed, 95% CI) | 2.24 [‐0.24, 4.73] |
|
| 9 Change in pm PEF Show forest plot | 4 | 4299 | L/min (Fixed, 95% CI) | 0.25 [‐0.80, 1.30] |
|
| 10 Change in FEV1 Show forest plot | 4 | 4845 | L (Fixed, 95% CI) | 0.00 [‐0.02, 0.02] |
|
| 11 Change in FEV1 predicted (%) Show forest plot | 1 | | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
|
| 12 Change in rescue medication use Show forest plot | 3 | 3469 | Puffs/d (Fixed, 95% CI) | ‐0.06 [‐0.13, 0.02] |
|
| 13 Change in daytime symptoms Show forest plot | 3 | 3464 | Symptoms (Fixed, 95% CI) | ‐0.02 [‐0.06, 0.03] |
|
| 14 Change in symptom‐free days Show forest plot | 2 | 3027 | Symptoms (Fixed, 95% CI) | 1.25 [‐1.18, 3.67] |
|
| 15 Change in nocturnal awakenings Show forest plot | 1 | | Symptoms (Fixed, 95% CI) | Totals not selected |
|
| 16 Adverse events Show forest plot | 3 | 3547 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.88, 1.15] |
|
| 17 Headache Show forest plot | 4 | 2916 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.82, 1.43] |
|
| 18 Candidiasis Show forest plot | 2 | 1272 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.68, 4.00] |
|
| 19 Upper respiratory tract infection Show forest plot | 2 | 1644 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.81, 1.47] |
|
| 20 Dysphonia Show forest plot | 3 | 2669 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.87, 2.43] |
|
| 21 Rhinitis Show forest plot | 1 | | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
|
| 22 Throat irritation Show forest plot | 2 | 1644 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.82, 2.35] |
|
| 23 Cough Show forest plot | 1 | | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
|
| 24 Tremor Show forest plot | 1 | | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
|
| 25 Withdrawals Show forest plot | 5 | 5082 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.78, 1.20] |
|
| 26 Withdrawals (adverse events) Show forest plot | 5 | 5082 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.60, 1.46] |
|
| 27 Withdrawals (lack of efficacy) Show forest plot | 1 | | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
|