Morfina oral para el dolor por cáncer
Information
- DOI:
- https://doi.org/10.1002/14651858.CD003868.pub4Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 22 April 2016see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Pain, Palliative and Supportive Care Group
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
PW initiated the review, carried out the searches, agreed on included and excluded trials, extracted the data, and wrote the text. RAM worked on the updated review for 2013 by extracting data, performing some analyses, and helped re‐write the text. BW provided clinical oversight. PW undertook the 2015 update. The review will be stabilised for five years as it is unlikely that new evidence with the potential to change the conclusions will be available in the near future.
Sources of support
Internal sources
-
Oxford Pain Research funds, UK
External sources
-
Department of Health, UK
-
The National Institute for Health Research (NIHR), UK
For the 2015 update: NIHR Cochrane Programme Grant: 13/89/29 ‐ Addressing the unmet need of chronic pain: providing the evidence for treatments of pain.
Declarations of interest
PW none known.
RAM has received institutional grant support from RB relating to individual patient level analyses of trial data on ibuprofen in acute pain and the effects of food on drug absorption of analgesics (2013), and from Grünenthal relating to individual patient level analyses of trial data regarding tapentadol in osteoarthritis and back pain (2015). He has received honoraria for attended boards with Menarini concerning methods of analgesic trial design (2014), with Novartis (2014) about the design of network meta‐analyses, and RB on understanding pharmacokinetics of drug uptake (2015).
BW is a specialist palliative care practitioner and manages patients with cancer pain.
This review was identified in a 2019 audit as not meeting the current definition of the Cochrane Commercial Sponsorship policy. At the time of its publication it was compliant with the interpretation of the existing policy. As with all reviews, new and updated, at update this review will be revised according to 2020 policy update.
Acknowledgements
Thanks to Sylvia Bickley for developing the original search strategies for this review. Thanks also to Jayne Rees and Jodie Barden who were authors on the first published version of this review, and Henry McQuay who was an author the first two published versions.
Cochrane Review Group funding acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane PaPaS Group. Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health.
The 2015 review update was developed as part of the NIHR Cochrane Programme Grant: 13/89/29 ‐ Addressing the unmet need of chronic pain: providing the evidence for treatments of pain.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Apr 22 | Oral morphine for cancer pain | Review | Philip J Wiffen, Bee Wee, R Andrew Moore | |
| 2013 Jul 23 | Oral morphine for cancer pain | Review | Philip J Wiffen, Bee Wee, R Andrew Moore | |
| 2007 Oct 17 | Oral morphine for cancer pain | Review | Philip J Wiffen, Henry J McQuay | |
| 2003 Oct 20 | Oral morphine for cancer pain | Review | Philip J Wiffen, J E Edwards, J Barden, Henry HJ McQuay, Jayne Rees | |
Differences between protocol and review
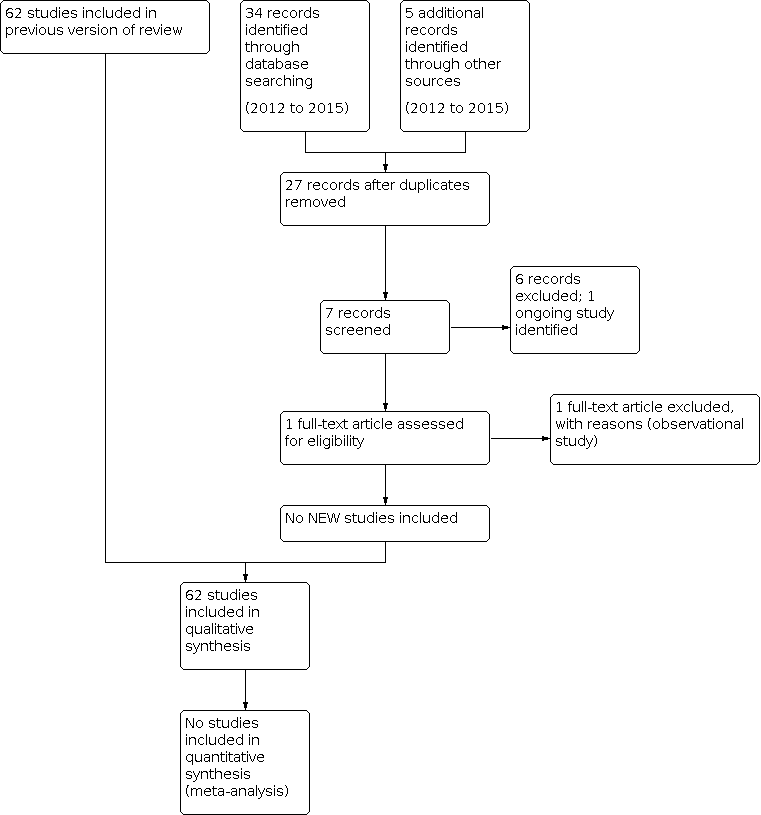
This review has developed considerably since the protocol was first published in 2002. Since that date the requirements for Cochrane reviews have changed significantly. For this update and the previous version we have added an outcome of no worse than mild pain and 'Risk of bias' tables and graphs.
Notes
A new search within two years is not likely to identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be re‐assessed for updating in five years. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Assessed for updating in 2021
In February 2021 we did not identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised for five years following discussion with the authors and editors. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Child; Humans;
PICOs

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Percentage of participants with oral morphine reporting no worse than mild pain. The size of the symbol is proportional to the size of the sample

Percentage of participants with oral morphine reporting an outcome equivalent to treatment success. The size of the symbol is proportional to the size of the sample