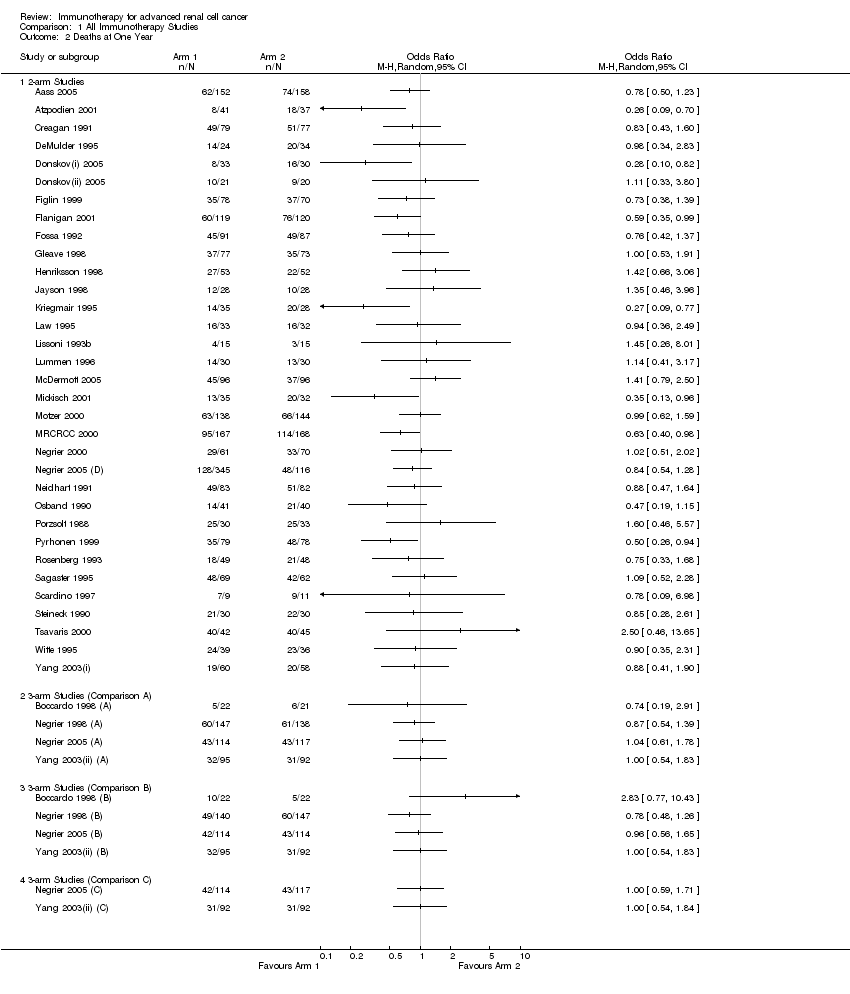
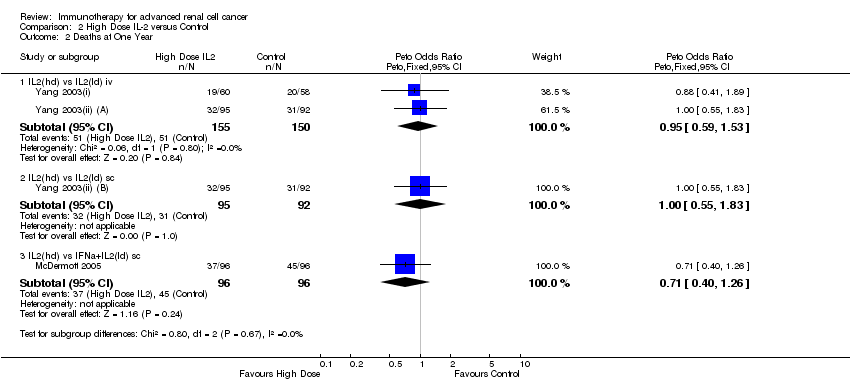
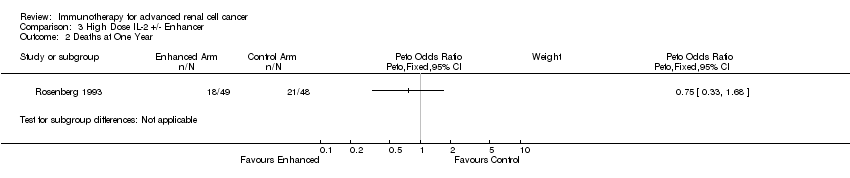
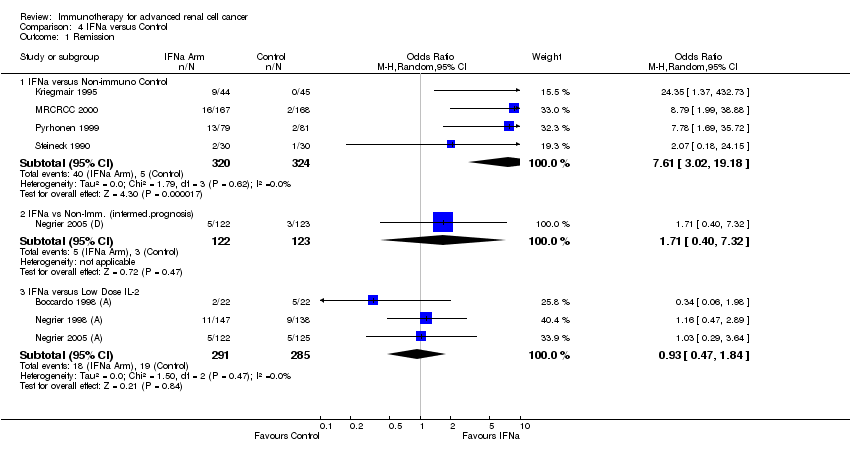
| Aass 2005 | 1,2 | | | s.c. 3‐9 MU daily | 13CRA |
| Aass 2005 | | | | | |
| Atzpodien 2001 | 1 | 5‐20 | s.c. 10 MU/m2 b.i.d. d3‐5 w1+4; 5 MU/m2 d1+3+5 w2+3 | s.c. 5 MU/m2 d1 w1+4, d1,3,5 w2+3; 10 MU/m2 d1,3,5 w5‐8 | 5‐fluorouracil |
| Atzpodien 2001 | | | | | |
| Atzpodien 2004 | 1,2 | 5‐20 | s.c. 10 MU/m2 b.i.d. d3‐5 w1+4; 5 MU/m2 d1+3+5 w2+3 | s.c. 5 MU/m2 d1 w1+4, d1,3,5 w2+3; 10 MU/m2 d1,3,5 w5‐8 | 5FU (arms 1&2); 13CRA (arm 1) |
| Atzpodien 2004 | 3 | | | s.c. 5 MU/m2 d1 w1, 10 MU/m2 d1,3,5 w2‐8 | vinblastine |
| Boccardo 1998 | 1,3 | | | i.m. 6 MU/m2 t.i.w. | |
| Boccardo 1998 | 2,3 | 18 | c.i.v.i 18 MU/m2 /24hr d1‐4 w1,3,7,9,13,17,21,25 | | |
| Brinkmann 2001 | 1 | 4.5‐9 | s.c. 9 MU/m2 d3‐5 w1+4; 4.5 MU/m2 d1+3+5 w2+3 | s.c. 4.5 MU/m2 d1 w1+4, d1,3,5 w2+3; 9 MU/m2 d1,3,5 w5‐8 | |
| Brinkmann 2001 | | | | | |
| Buzogany 2001 | 1,2 | | | s.c. 10 MU t.i.w. x12w | vinblastine (arm 1) |
| Buzogany 2001 | | | | | |
| Creagan 1991 | 1,2 | | | i.m. 20 MU/m2 t.i.w. | ASA (arm 1) |
| Creagan 1991 | | | | | |
| DeMulder 1995 | 1,2 | | | s.c. 10 MU/m2 b.i.w. | IFN‐gamma (arm 1) |
| DeMulder 1995 | | | | | |
| Dexeus 1989 | 1 | | | i.m. 3 MU/m2 d1,15, 5 MU/m2 d2,16, 10 MU/m2 d3,17‐19 q7weeks | combination chemotherapy |
| Dexeus 1989 | | | | | |
| Donskov(i) 2005 | 1,2 | 10.5 | s.c. 18 MU d1‐5, 8‐12, 15‐19 q5w | | histamine dihydrochloride |
| Donskov(i) 2005 | | | | | |
| Donskov(ii) 2005 | 1,2 | 10.5 | s.c. 18 MU d1‐5, 8‐12, 15‐19 q5w | | histamine dihydrochloride |
| Donskov(ii) 2005 | | | | | |
| Edsmyr 1985 | 1 | | | i.m. 3 MU daily | |
| Edsmyr 1985 | | | | | |
| Figlin 1999 | 1,2 | 5 | c.i.v.i. 5 MU/m2/24hr d1‐4 w1‐4 | | TIL (arm 1); placebo (arm 2) |
| Figlin 1999 | | | | | |
| Flanigan 2001 | 1,2 | | | s.c. 5 MU/m2 t.i.w. | nephrectomy (arm 1) |
| Flanigan 2001 | | | | | |
| Foon 1988 | 1,3 | | | s.c. 2 MU/m2 t.i.w. | |
| Foon 1988 | | | | | |
| Fossa 1992 | 1,2 | | | i.m. 18 MU t.i.w. | |
| Fossa 1992 | | | | | |
| Fujita 1992 | 1 | | | i.m. 3 MU 6d/week | |
| Fujita 1992 | 2 | | | i.m. 1 MU 6d/week | |
| Henriksson 1998 | 1 | 4.8‐14.4 | s.c. 4.8 MU/m2 q8h d1,22 + q12h d2,23 + 2.4 MU/m2 q12h d3‐5, 8‐12, 15‐19, 24‐26, 29‐33, 36‐40 | s.c. 3 MU/m2 d3,5,24,26 + 6 MU/m2 d8,10,12,15,17,19,29,31,33,38,40 | tamoxifen |
| Henriksson 1998 | | | | | |
| Jayson 1998 | 1 | 10.5 | s.c. 18 MU d1‐5 w1‐3 q4w | s.c. 9 MU t.i.w. w1‐3 q4w | |
| Jayson 1998 | 2 | 10.5 | s.c. 18 MU d1‐5 w1‐3 q4w | | |
| Kinouchi 2004 | 1,2 | | | 5 MU 5d/week (route not stated) | cimetidine (arm 1) |
| Kinouchi 2004 | | | | | |
| Kirkwood 1985 | 1 | | | i.m. 10 MU daily | |
| Kirkwood 1985 | 2 | | | i.m. 1 MU daily | |
| Kriegmair 1995 | 1 | | | s.c. 8 MU t.i.w. | |
| Kriegmair 1995 | | | | | |
| Law 1995 | 1,2 | 3 | c.i.v.i 3 MU/m2/24hr d1‐5 w1‐4 | | LAK cells (arm 1) |
| Law 1995 | | | | | |
| Lissoni 1993b | 1 | 3.5 | s.c. 3 MU b.i.d. 1‐5 x6w x2 cycles, then 6d/month | s.c. 5 MU/m2 t.i.w. | |
| Lissoni 1993b | 2 | 3.5 | s.c. 3 MU b.i.d. 1‐5 x6w x2 cycles, then 6d/month | | |
| Lissoni 2000 | 1,2 | 6 | s.c. 3 MU b.i.d. 1‐5 x4w | | morphine; melatonin (arm 1) |
| Lissoni 2000 | | | | | |
| Lummen 1996 | 1 | 2.4‐14.4 | s.c. 4.8 MU/m2 q8hx4 d1,22 + q12hx2 d2,23; 2.4 MU/m2 q12hx2 with each IFNa | s.c. 3 MU/m2 d3,5,24,26; 6 MU/m2 d8,10,12,15,17,19,29,31,33,36,38,40 | |
| Lummen 1996 | | | | | |
| McCabe 1991 | 1,2 | 19.5 | i.v. 0.1 MU/kg t.i.d. d1‐5,11‐15 | | LAK (arm 1) |
| McCabe 1991 | | | | | |
| McDermott 2005 | 1 | 5‐15 | s.c. 5 MU/m2 q8hx3 d1 then dailyx5 x4w, q6w | s.c. 5 MU/m2 t.i.w. x4w q6w | |
| McDermott 2005 | 2 | 69 | i.v. 0.6 MU/kg t.i.d. d1‐5,15‐19 q12w | | |
| Mickisch 2001 | 1,2 | | | s.c. 5 MU/m2 t.i.w. | nephrectomy (arm 1) |
| Mickisch 2001 | | | | | |
| Motzer 2000 | 1,2 | | | s.c. 3 MU d1‐7, 6 MU d8‐14, 9 MU d15‐ | 13CRA (arm 1) |
| Motzer 2000 | | | | | |
| Motzer 2001 | 2 | | | s.c. 9 MU t.i.w. | |
| Motzer 2001 | | | | | |
| MRCRCC 2000 | 1 | | | s.c. 10 MU t.i.w. x12w | |
| MRCRCC 2000 | | | | | |
| Muss 1987 | 1 | | | i.v. 30 (‐50) MU/m2 d1‐5 q3w | |
| Muss 1987 | 2 | | | s.c. 2 (‐10) MU/m2 t.i.w. | |
| Naglieri 1998 | 1 | 2.6‐5.3 | s.c. 9 MU d1,2 then 4.5 MU d3‐5 week 1 + d1‐5 wk2‐6 | s.c. 3 MU d1,3,5 x6w | |
| Naglieri 1998 | 2 | 2.6‐5.3 | s.c. 9 MU d1,2 then 4.5 MU d3‐5 week 1 + d1‐5 wk2‐6 | | 4‐epirubicin |
| Negrier 1998 | 1 | | | s.c. 18 MU/m2 t.i.w. | |
| Negrier 1998 | 2,3 | 18 | c.i.v.i. 18 MU/m2/24hr d1‐5 w1,3,7,9,13,17,21,25 | arm3 only: s.c. 6 MU/m2 t.i.w. w1,3,7,9.13,17,21,25 | |
| Negrier 2000 | 1,2 | 5.3 | s.c. 9 MU d1‐6 w1,3,5,7 | s.c. 6 MU d1,3,5 w1,3,5,7 | 5FU (arm 1) |
| Negrier 2000 | | | | | |
| Negrier 2005 | 1,3 | | | s.c. 9 MU t.i.w. | |
| Negrier 2005 | 2,3 | 10.5 | s.c. 18 MU 5d/week x4w | | |
| Negrier 2005 (D) | 4 | | | | medroxyprogesterone |
| Negrier 2005 (D) | | | | | |
| Neidhart 1991 | 1,2 | | | i.m. 3‐5‐20‐20‐20 MU/m2 d1‐5 q2w | vinblastine (arm 1) |
| Neidhart 1991 | | | | | |
| Porzsolt 1988 | 1,2 | | | s.c. 2MU d1‐5 x8‐12w, then once weekly | medroxyprogesterone (arm 1) |
| Porzsolt 1988 | | | | | |
| Pyrhonen 1999 | 1 | | | s.c. 18 MU t.i.w. | vinblastine |
| Pyrhonen 1999 | | | | | |
| Quesada 1985 | 1 | | | i.m. 20 MU daily | |
| Quesada 1985 | 2 | | | i.m. 2 MU daily | |
| Radosavljevic 2000 | 1,2 | | | 6 MU t.i.w. (route not stated) | vinblastine; MPA (arm 1) |
| Radosavljevic 2000 | | | | | |
| Rosenberg 1993 | 1,2 | 83 | i.v. 0.72 MU/kg q8h x4‐5d, x2 cycles | | LAK cells (arm 1) |
| Rosenberg 1993 | | | | | |
| Sagaster 1995 | 1,2 | | | s.c. 5 MU d1‐5 | coumarin, cimetidine (arm 1) |
| Sagaster 1995 | | | | | |
| Scardino 1997 | 1 | 3.5‐7 | s.c. 18 MU d1‐3 preop + 6 MU d1‐5 x6w postop | | nephrectomy |
| Scardino 1997 | 2 | 3.5‐7 | s.c. 6 MU d1‐5 x6w postop | | nephrectomy |
| Steineck 1990 | 1 | | | i.m. 10(‐20) MU/m2 t.i.w. | |
| Steineck 1990 | | | | | |
| Tannir 2004 | 1 | | | s.c. 5 MU daily | |
| Tannir 2004 | 2 | | | s.c. 0.5 MU b.i.d. | |
| Tsavaris 2000 | 1 | | | s.c. 5 MU t.i.w. | vinblastine |
| Tsavaris 2000 | 2 | | | s.c. 15 MU t.i.w. | |
| Weiss 1992 | 1 | 15 | c.i.v.i. 1 mg (3‐5 MU)/m2/24hr x 4.5d, 1.25 mg/m2/24hr d11‐15 | | LAK cells |
| Weiss 1992 | 2 | 4‐5 | i.v. 33 mcg/kg q8h x14 doses d1‐5,11‐15 | | LAK cells |
| Witte 1995 | 1,2 | 6 | i.v. 6 MU/m2 t.i.w. x4w then 2 weeks out of 4 | | interferon‐beta (arm 1) |
| Witte 1995 | | | | | |
| Yang 1995 | 1 | 83 | i.v. 0.72 MU/kg q8h x15(max) week 1, then PEG‐IL2 3(‐6) MU/kg weekly x3, q2months | | |
| Yang 1995 | 2 | 83 | i.v. 0.72 MU/kg q8h x15(max) week x2, q2months | | |
| Yang 2003(i) | 1 | 83 | i.v. 0.72 MU/kg q8h x4‐5d, x2 cycles | | |
| Yang 2003(i) | 2 | 8.3 | i.v. 0.072 MU/kg q8h x4‐5d, x2 cycles | | |
| Yang 2003(ii) | 3 | 5‐10 | s.c. 0.25 MU/kg d1‐5, then 0.125 MU/kg x5d weeks 2‐6 | | |
| Yang 2003(ii) | | | | | |